A technical proposal is a document that outlines the details of a project or solution that is being proposed. It is often used in the fields of engineering, construction, and technology, and it is designed to provide a clear and concise description of the project, as well as the benefits and costs associated with it.
There are many different topics that can be addressed in a technical proposal, depending on the nature of the project and the needs of the client. Some common topics that are often included in technical proposals include:
Project overview: This section provides a high-level description of the project, including the objectives, scope, and timeline.
Problem statement: This section outlines the problem or challenge that the project is designed to address. It should clearly describe the issue and explain why it is important to address it.
Solution: This section outlines the proposed solution to the problem or challenge. It should provide a detailed description of the proposed solution and explain how it will address the problem or challenge.
Methodology: This section outlines the approach that will be taken to implement the proposed solution. It should include details on the materials, tools, and processes that will be used, as well as any key milestones or deliverables.
Benefits: This section should outline the benefits that will be realized as a result of the project. This could include cost savings, increased efficiency, improved performance, or other positive outcomes.
Costs: This section should outline the costs associated with the project, including materials, labor, and any other expenses. It should also include any contingencies or risk management strategies.
Conclusion: This section should summarize the key points of the proposal and explain why the proposed solution is the best fit for the client's needs.
Overall, a well-written technical proposal should provide a clear and concise overview of the project and its benefits, as well as a detailed plan for how it will be implemented. It should be tailored to the specific needs of the client and should clearly demonstrate the value and benefits of the proposed solution.
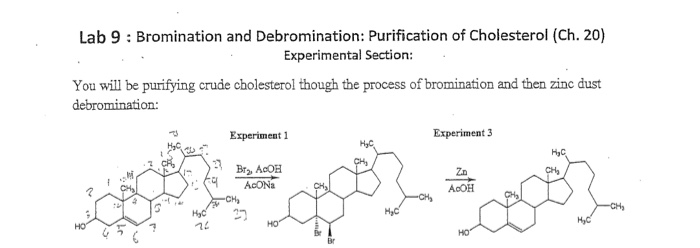
Debromination and Bromination of Cholesterol

The remaining solvent was decanted and the synthesized cholesterol transferred and allowed to dry in the hood for 20 minutes. After the cholesterol solution was cooled, the flask was clamped to the ring on the hot plate as shown in figure 4 and stirred without heating. Negative results were observed for both the commercial cholesterol and 1-chlorobutane. About 1 mL of chloroform CHCl3 was added to each tube and vortex to completely dissolve all solids. The difference in the distance traveled and the Rf values of the samples commercial and synthesized cholesterol were pure. Figure 3: Set-up for bromination reaction Note: the actual reaction mixture is not blue.
Bromination and Debromination of Cholesterol (1).docx

When the cholesterol dibromide starts to crystalize while it is still on the heat it will be taken down from the head and it will be placed in the ice bath and stir it. About 20 mL of the t-butylmethyl ether — acetic acid solution was then dispensed in a clean 50 mL Erlenmeyer flask which was clamped to a ring stand and allowed to cool in the ice bath. The observation for this reaction was recorded in the notebook. References Feiser, Louis, and Williamson, Kenneth. On the other hand, the silver nitrate reagent reacted with 2 o and 3° alkyl halides through an S N1 mechanism.
Examining The Bromination And Debromination Of Cholesterol Biology Essay

The percent yield was calculated to be 26. The percent yield was calculated to be 26. About 30 mg of the commercial cholesterol starting material was added to tube A; ~30 mg of dibromocholesterol to tube B; ~30 mg of the synthesized cholesterol product to tube C; ~0. Crude cholesterol is isolated from natural sources and various methods have been used in its purification. A water bath was then set up on the hotplate in the hood. Because of this agreement in melting point, it was confirms the purity of the synthesized cholesterol.
Bromination
On the other hand, cholestanal does not react with bromine and the other two contaminants are dehydrogenated by bromine leading to formation of soluble dienes and trienes respectively. The formation of the precipitate also indicated a positive result. Negative results were observed for both the commercial cholesterol and 1-chlorobutane Zubrick 38. Get Help With Your Essay If you need assistance with writing your essay, our professional essay writing service is here to help! These were Sodium Iodide test, Silver Nitrate test, and Sulfuric acid test. In addition, the presence of double bonds in dibromocholesterol in form of alkene makes it possible for the formation of a fluorescent green sulfuric acid layer and a red chloroform layer when reacted with sulfuric acid Landgrebe 78. The plate was spotted with each solution and developed by placing the plate using 30% ethyl acetate: 70% hexane as the mobile phase.
Debromination and Bromination of Cholesterol

A purification step such as solvent washing or crystallization is carried out to separate the solid from the impurities. The Erlenmeyer flask contain the reaction solution was inserted into the water bath and clamped as shown below. The TLC analysis of cholesterol and dibromocholesterol was performed by obtaining a silica gel TLC plate and setting it up to run TLC analysis on solutions A-C above. Then the cooling fan was plugged in. All Answers ltd, 'Examining The Bromination And Debromination Of Cholesterol Biology Essay' UKEssays. Each of these tests is selective for a specific functional group.
Bromination and debromination of blog.sigma-systems.com
Thus due to differences in their molecular structures, the components moved up the paper at different rates. The flask was removed from the water bath after all the cholesterol was completely dissolved and allowed to cool to room temperature. Get Help With Your Essay If you need assistance with writing your essay, our professional essay writing service is here to help! After crystals appears remove the solution gives some time for it to be in room temperature after that put it in ice bath, collect the crystals and wash it with cold methanol and find the melting point. It is also a major precursor for most steroid hormones. Tubes A-C did the TLC as well. In addition, synthesized cholesterol also gave a positive result with sodium iodide test as the solution turned in the formation of chunky and yellow solution.
Debromination of cholesterol dibromide
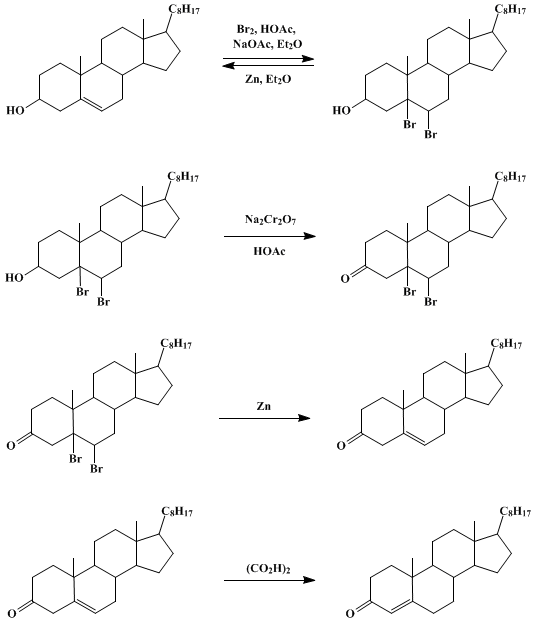
The remaining solvent was decanted and the synthesized cholesterol transferred and allowed to dry in the hood for 20 minutes. The addition of zinc dust successfully removed the bromides from the cholesterol structure to produce a purified cholesterol. In addition, about 3 mL of acetone was added to each tube to completely dissolve all the compounds. In addition, about 3 mL of acetone was added to each tube to completely dissolve all the compounds. The solid was then allowed to dry with the vacuum on for about 5 minutes. From simple essay plans, through to full dissertations, you can guarantee we have a service perfectly matched to your needs. The absence of other spots on the TLC plate confirms that there were no contaminations present in the sample.
Lab 6 debromination
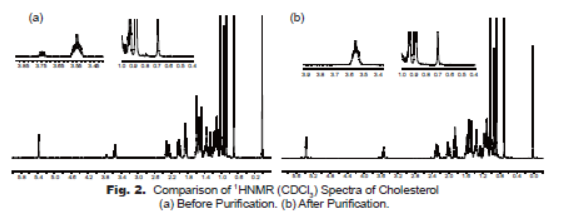
NaI test showed a positive response as color changed to yellow. A precipitate solution formed almost immediately. Peak CI 2 and CI 3 mark the place where C-H bonds appeared on the initial cholesterol sample, and their disappearance confirms a successful bromination reaction. Use discount The two layers formed were then separated as water layers and organic ether layers. The leading edge of the solvent is known as the solvent front. The sodium iodide reagent reacted with 1° and 2° alkyl halides through an S N2 mechanism.






