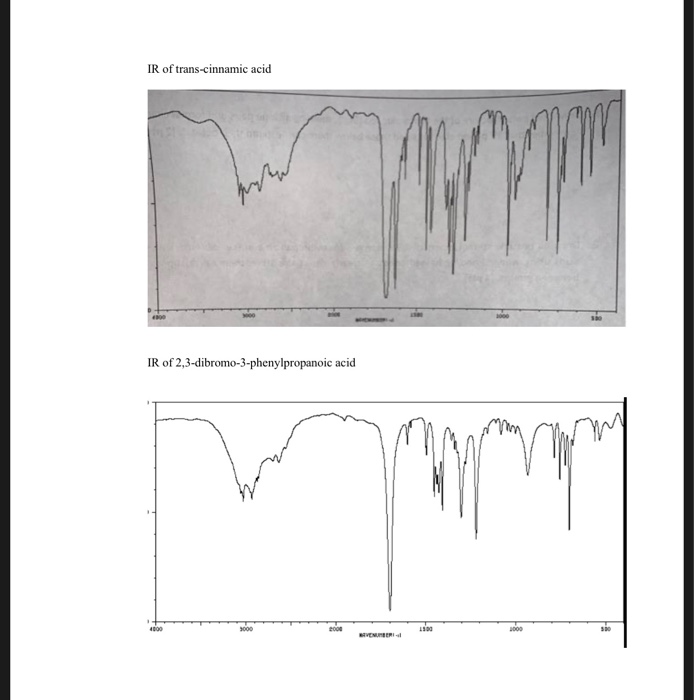
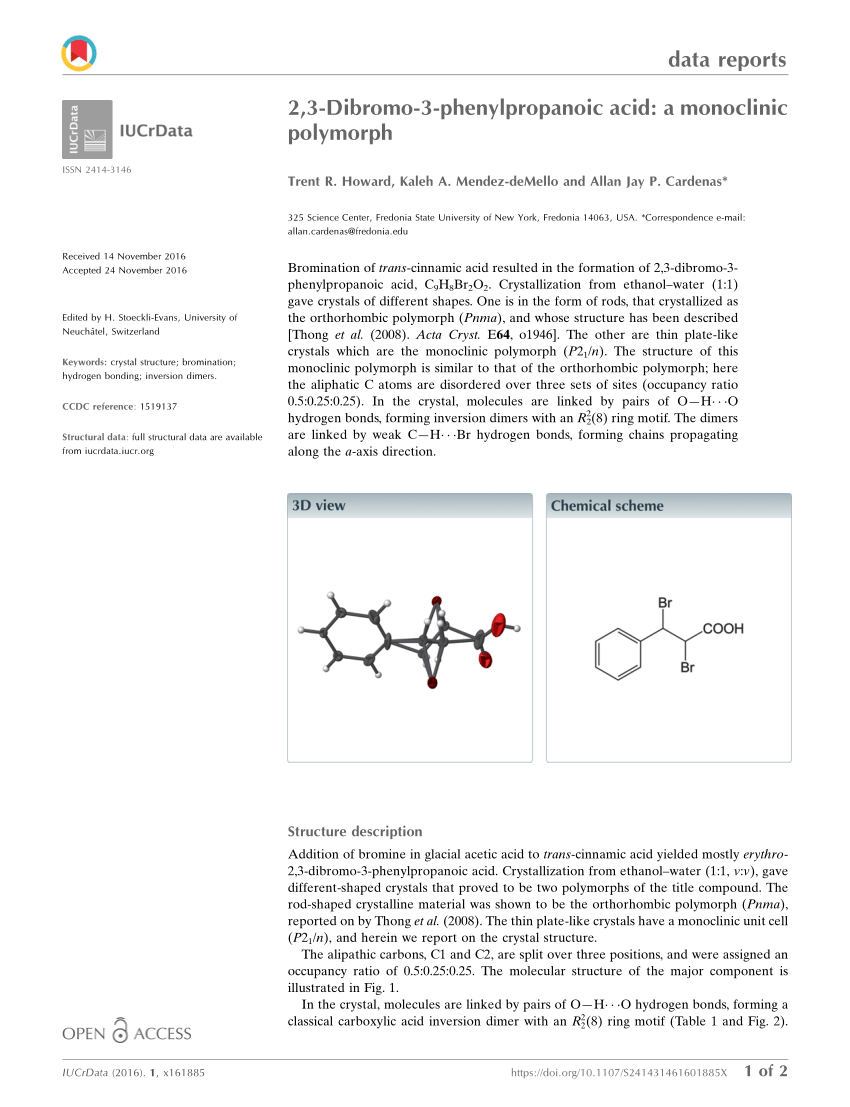
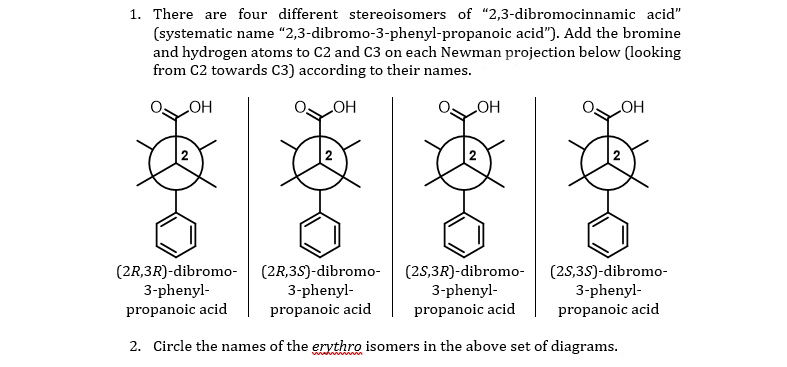
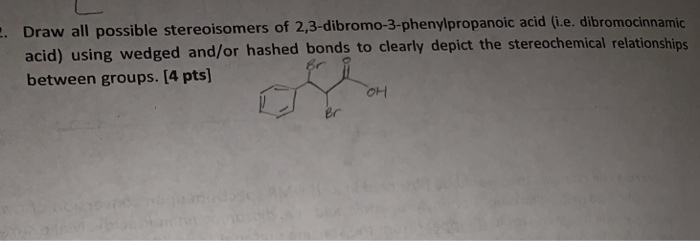
Dibromocinnamic acid, also known as 2,3-dibromocinnamic acid or cinnamic acid 2,3-dibromide, is a chemical compound with the molecular formula C9H7Br2O2. It is a white, crystalline solid that is soluble in organic solvents and has a melting point of around 199-202 °C.
Dibromocinnamic acid is mainly used as an intermediate in the synthesis of various organic compounds, such as fragrances, dyes, and pharmaceuticals. It can also be used as a crosslinking agent in the production of polymers.
One of the main methods for the synthesis of dibromocinnamic acid is the bromination of cinnamic acid, which can be achieved using hydrogen bromide or bromine in the presence of a suitable catalyst. Other methods for synthesizing dibromocinnamic acid include the reaction of cinnamic acid with dibromomethane or the reaction of cinnamic acid with bromine in the presence of a palladium catalyst.
Dibromocinnamic acid has several important physical and chemical properties that make it useful in various applications. For example, it is a strong acid, with a pKa value of around 2.6, which means it can easily donate protons to water and other solvent molecules. It also has a high affinity for water, with a solubility of around 15 g/L in water at room temperature.
Despite its potential uses, dibromocinnamic acid can also be toxic and harmful to humans and the environment. It is classified as a toxic chemical by the European Chemicals Agency (ECHA) and is listed as a Category 2 environmental hazard according to the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). This means that it may cause harmful effects on the environment if released into the environment in large amounts. As a result, it is important to handle dibromocinnamic acid with caution and in accordance with appropriate safety guidelines.
In conclusion, dibromocinnamic acid is a chemical compound with a wide range of potential uses as an intermediate in the synthesis of various organic compounds. However, it is also toxic and harmful to humans and the environment, and must be handled with caution.