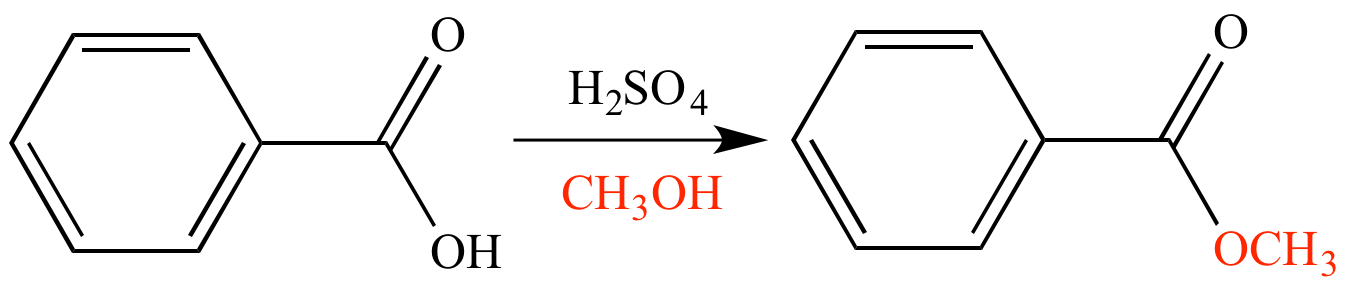
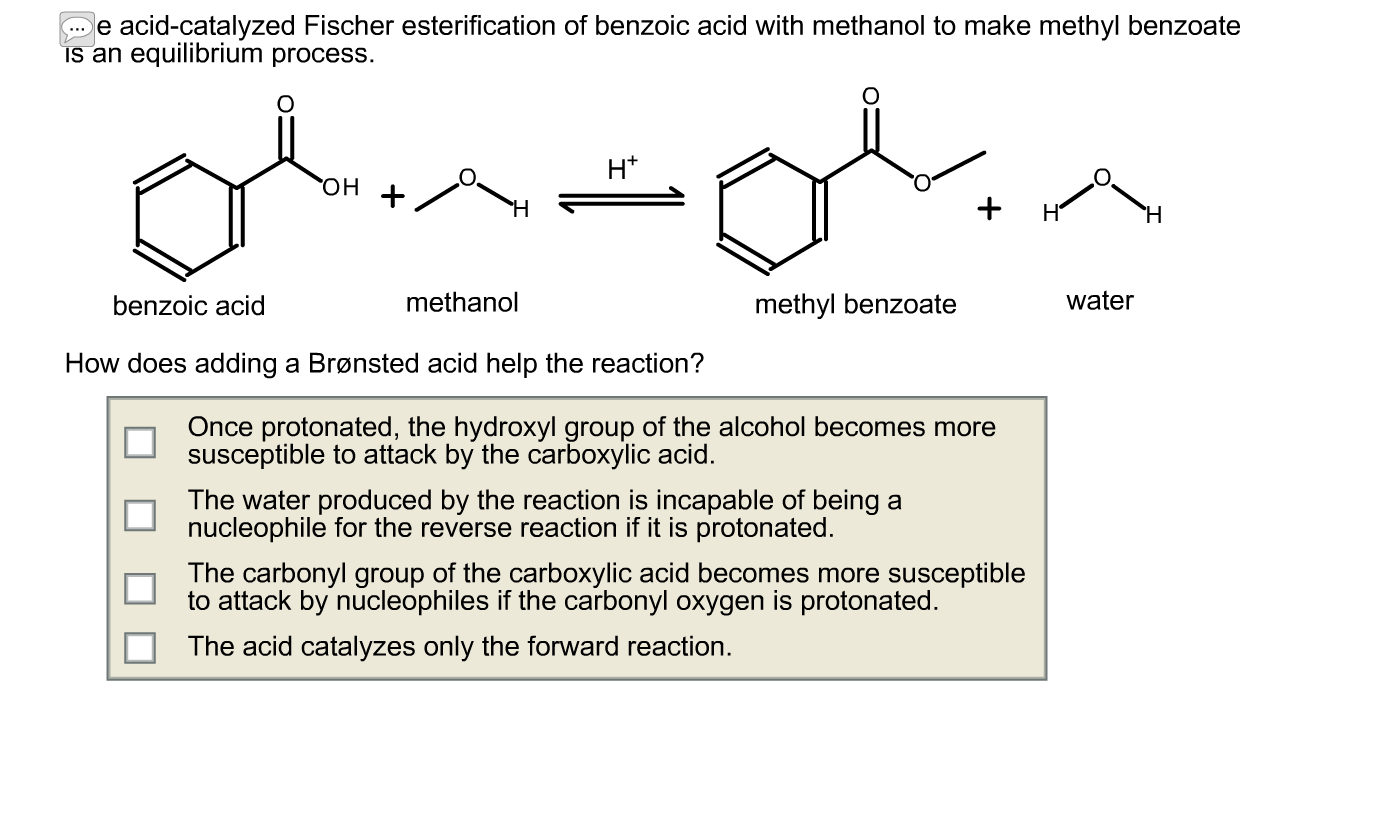
Fischer esterification is a chemical reaction that is commonly used to synthesize esters from carboxylic acids and alcohols. This reaction is named after the German chemist Emil Fischer, who first described the reaction in the late 19th century.
One example of the Fischer esterification reaction is the synthesis of benzoic acid esters. Benzoic acid is a common carboxylic acid that is found in many natural and synthetic products, including food additives, fragrances, and pharmaceuticals. By reacting benzoic acid with an appropriate alcohol, it is possible to synthesize a wide variety of benzoic acid esters, each with unique properties and applications.
The Fischer esterification reaction is typically carried out in the presence of an acid catalyst, such as sulfuric acid or hydrochloric acid. The catalyst helps to protonate the carboxylic acid, which makes it more reactive and able to react with the alcohol. The reaction is usually carried out at high temperatures, often around the boiling point of the solvent, to promote the reaction and drive it to completion.
In the case of benzoic acid esters, the resulting esters can be used for a variety of purposes. For example, benzyl benzoate is used as a fixative in perfumes, while ethyl benzoate is used as a flavorant in food and beverages. Other benzoic acid esters, such as butyl benzoate and pentyl benzoate, are used as plasticizers in the production of plastics and rubber.
Overall, the Fischer esterification reaction is a useful and widely used method for synthesizing a variety of esters, including benzoic acid esters. By carefully selecting the reactants and adjusting the reaction conditions, it is possible to produce esters with a wide range of properties and applications.