Free radical bromination is a chemical reaction in which bromine atoms are added to a molecule through the process of radical substitution. This reaction occurs when a molecule with a carbon-hydrogen bond is exposed to bromine and light or heat, which causes the bromine molecule to dissociate into atoms. These atoms then react with the carbon-hydrogen bonds, replacing the hydrogen atoms with bromine atoms.
The process of free radical bromination can be used to synthesize a wide range of compounds, including pharmaceuticals, plastics, and dyes. It is an important tool in the field of organic chemistry, as it allows chemists to selectively modify the structure of molecules and tailor their properties to meet specific needs.
One of the key advantages of free radical bromination is its ability to selectively target specific bonds within a molecule. This allows chemists to control the location of the bromine atoms within the molecule, which can have a significant impact on the properties of the resulting compound. For example, bromination of an aromatic compound can often result in the introduction of new functional groups, which can significantly alter the reactivity of the molecule.
There are several different methods for carrying out free radical bromination, including the use of light or heat to initiate the reaction, or the use of a chemical initiator such as a peroxide or a halogenated hydrocarbon. The choice of method will depend on the specific requirements of the reaction, such as the nature of the starting material and the desired product.
Despite its usefulness, free radical bromination also has some limitations. One of the main challenges is that it can produce a range of different brominated products, depending on the reaction conditions and the structure of the starting material. This can make it difficult to predict the outcome of the reaction and can require careful optimization of the reaction conditions in order to obtain the desired product.
Overall, free radical bromination is a powerful tool for the synthesis of a wide range of compounds and continues to be an important area of research in the field of organic chemistry.
9.4 Chlorination vs Bromination

This is called the initiation step because it initiates the reaction. But as the reaction mixture is heated, the proportion of those molecules increases. As expected, reaction rates increase with increasing substitution at the benzylic position, but this paper provides experimental evidence for that. Due to this fact we can tell since they were the fastest reacting, they can be associated with the fact that they were more than likely secondary and primary benzylic carbons due to their excellent stability and fast reactions. In this step, the sigma bond in molecular bromine is homolytically cleaved to form the free radicals.
Free Radical Halogenation Mechanism
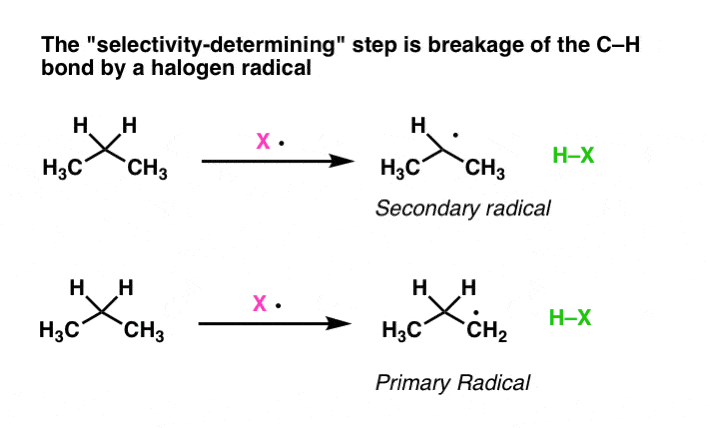
Is there a factor that prevents radical bromination at several carbons in an alkane? Even when they react, the electrons still usually move in pairs. Drawing Lewis structures for radicals is not that different from what we covered above. When reacting with a molecular bromine, more free-radical bromine is reacted. The first step is the abstraction of the hydrogen atom from the tertiary carbon a tertiary carbon is a carbon that is attached to three other carbon atoms Note that these are not protons H+ ions that are being abstracted, but actual hydrogen atoms since each hydrogen has one electron. The first propagation step uses up one of the products from initiation, and the second propagation step makes another one, thus the cycle can continue until indefinitely. The central carbon atom has a total of 4 valence electrons. The relative reaction rate of alkyl radicals for chlorinationhas been measured and has the approximate values of: Figure 9.
Free Radical Bromination of Alkanes

Think of the group 1A metals like Sodium and Lithium. For a reaction at 300K, we can calculate RT using the gas constant 1. The radical chain mechanism is characterized by three steps: initiation, propagation and termination. For an alkane with only one type of hydrogen, the problem of isomeric mixture can be prevented since only one product is produced. As a result, for the reaction of alkane with different hydrogen atoms, a mixture of isomeric monochlorinated products is obtained. One way to avoid this problem is to use a much higher concentration of methane in comparison to chloride.
Bromination

We will now briefly discuss types of bromination in detail. Step 3: Termination Termination takes place in three different ways. If you manage to take an electron away from its partner, you create one very unhappy, and thus very reactive , radical electron. Since radicals are, well, radical , they follow their own chain reaction system. Hendry Journal of the American Chemical Society 1963, 85 19 , 2976-2983 DOI: Significant solvent effects are observed in the free-radical photochlorination of benzylic compounds. To predict the relative amount of different chlorination product, we need to consider two factors at the same time: reactivityand probability. What this means is that for chlorination, the difference in activation energies between the two radical pathways i.
Free radical bromination [hν, Br2]

Bromination is a chemical reaction involving the reaction of a compound, and bromine results in bromine being added to the compound. Understanding this point begins with understanding the energy profile of these two different reactions chlorination and bromination. In fact, products of both the dark and light reactions are dictated by the stability of the intermediate. However, if the conditions are changed, so that either the reaction is taking place at high temperatures denoted by Δ or there is ultra violet irradiation, a product is formed, chloromethane CH 3Cl. The experimentally determined activation energies are all rather low, on the order of 0. The product formed after bromination will exhibit new properties from the initial reactant. At the far right of the scale are the most energetic molecules.
Radical Bromination

Take a look at the dissolving metal reduction alkyne to trans-alkene to see just how easily they want to give up their electrons. The next most stable carbocation form would be the tertiary aliphatic carbon, so we will infer that this is what has occurred in this case. Since radicals deal with just a single electron, we must use a very different arrow system to show how these radical reactions occur. Radicals, not so much! Reagent Table Compound M. I would appreciate your short answer. Since chlorine is a rather reactive reagent, it shows relative low selectivity, which means Cl 2 does not discriminate greatly among the different types of hydrogen atoms primary, secondary or tertiary in an alkane. For this step to occur energy must be put in, this step is not energetically favorable.








