Gravimetric analysis is a type of quantitative analytical method that involves the measurement of the mass of a substance in order to determine its concentration or purity. In the case of a chloride salt, such as sodium chloride or potassium chloride, gravimetric analysis can be used to determine the amount of chloride present in a sample.
There are several different approaches to conducting a gravimetric analysis of a chloride salt, but the most common method involves precipitation. Precipitation is the process of forming a solid from a solution by adding a reagent that reacts with the dissolved substance to produce a solid precipitate.
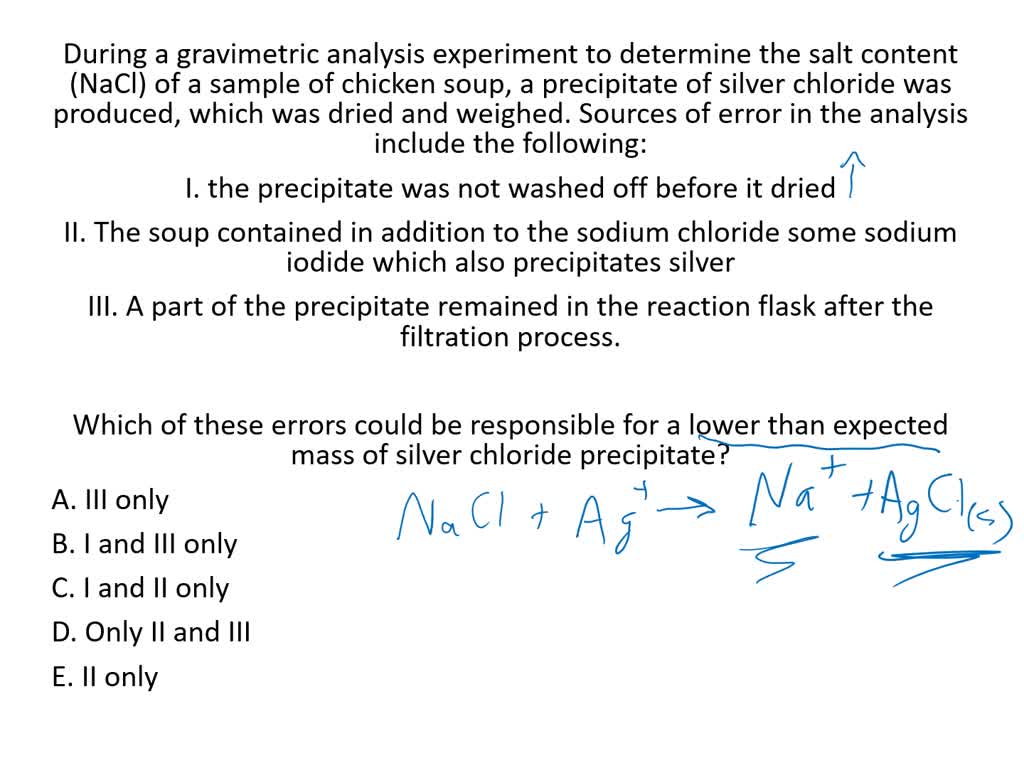
To begin the analysis, a sample of the chloride salt is dissolved in water to form a solution. Next, a reagent is added to the solution that reacts with the chloride ions to produce a solid precipitate. The most common reagent for this purpose is silver nitrate, which reacts with chloride ions to form silver chloride.
The precipitate is then allowed to settle to the bottom of the container, and the solution is decanted or filtered off. The precipitate is then dried and weighed to determine the mass of the chloride present in the sample.
The mass of the chloride in the sample can then be used to calculate the concentration of chloride in the solution. This can be done by comparing the mass of the chloride in the sample to the mass of the entire sample, or by using a standard curve.
In order to obtain accurate results, it is important to carefully control the conditions of the reaction and to carefully clean and dry the precipitate before weighing it. It is also important to use a high-quality balance or scale to accurately measure the mass of the precipitate.
Overall, gravimetric analysis is a useful and reliable method for determining the concentration of a chloride salt in a sample. By carefully controlling the conditions of the reaction and accurately measuring the mass of the precipitate, it is possible to obtain precise and accurate results.