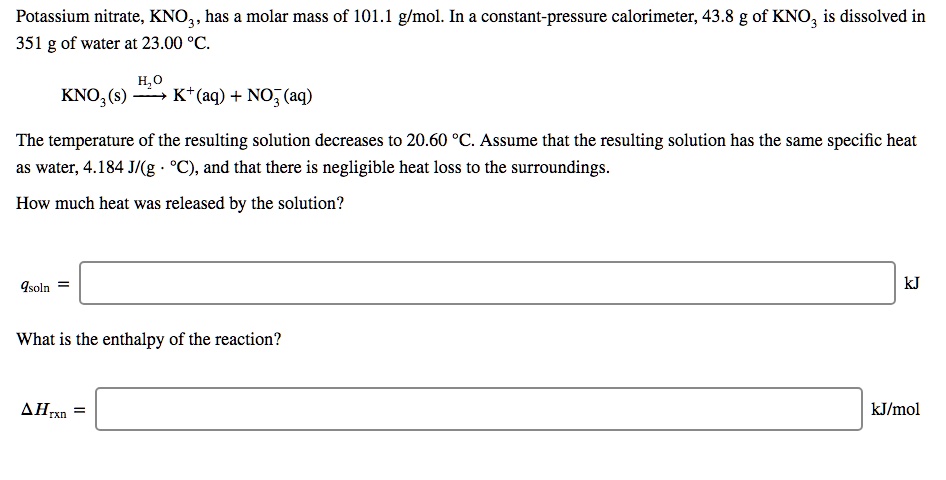
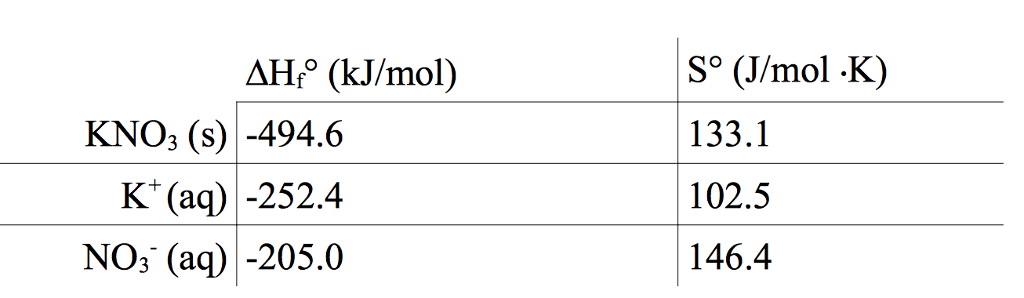
The heat of solution, also known as the enthalpy of solution, is the change in enthalpy that occurs when a substance is dissolved in a solvent to form a solution. In the case of potassium nitrate (KNO3), the heat of solution is the energy released or absorbed when the solid compound is dissolved in water.
The heat of solution can be either positive or negative, depending on the nature of the substance and the solvent. A positive heat of solution indicates that energy is released during the dissolution process, while a negative heat of solution indicates that energy is absorbed.
In the case of potassium nitrate, the heat of solution is positive, meaning that energy is released when the compound is dissolved in water. This is due to the fact that the solute-solvent interactions in the solution are more favorable than the solute-solute interactions in the solid compound. As a result, the potential energy of the system decreases when the solute is dissolved, leading to the release of energy in the form of heat.
The magnitude of the heat of solution for potassium nitrate can vary depending on the concentration of the solution and the temperature. Generally, the heat of solution increases with increasing concentration and decreasing temperature. This is due to the fact that the solute-solvent interactions become more favorable at higher concentrations and lower temperatures, leading to the release of more energy.
The heat of solution of potassium nitrate has a number of practical applications. For example, it is used in the production of fertilizers, explosives, and pyrotechnics, where the release of energy from the dissolution of the compound can be utilized. It is also used in the production of ice cream, where the release of heat during the dissolution of potassium nitrate helps to lower the temperature of the mixture, allowing it to freeze.
In conclusion, the heat of solution of potassium nitrate is a measure of the change in enthalpy that occurs when the compound is dissolved in water. It is a positive value, indicating that energy is released during the dissolution process. The magnitude of the heat of solution varies with concentration and temperature, and it has a number of practical applications in industries such as agriculture, explosives, and food production.