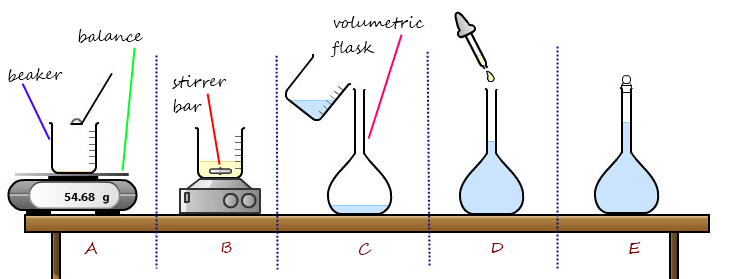
Standard solutions are solutions with a known concentration of a particular substance. They are used in a variety of industries as a reference for calibrating instruments and for performing quantitative analysis. Preparing standard solutions in industry involves a number of steps that ensure the accuracy and reliability of the solution.
The first step in preparing a standard solution is to select the appropriate solvent. The solvent should be a pure substance that is capable of dissolving the solute at the desired concentration. The solvent should also be stable, meaning it should not react with the solute or any other materials in the solution.
Once the solvent has been chosen, the next step is to accurately weigh out the required amount of solute. This is typically done using a balance or other precision weighing instrument. It is important to use the correct amount of solute in order to achieve the desired concentration of the solution.
Once the solute has been accurately weighed, it is added to a container and the solvent is added to the container. The solution is then stirred or shaken to ensure that the solute is fully dissolved in the solvent. The solution is then allowed to sit for a period of time to ensure that it has reached equilibrium.
After the solution has reached equilibrium, it is typically filtered to remove any undissolved particles. The solution is then transferred to a storage container, labeled, and stored until it is needed.
Standard solutions are typically prepared in small quantities and are used up relatively quickly. This ensures that the solution is as fresh and accurate as possible. It is important to follow proper laboratory techniques when preparing standard solutions, as even small errors can lead to inaccurate results.
In conclusion, preparing standard solutions in industry involves a number of steps to ensure the accuracy and reliability of the solution. These steps include selecting an appropriate solvent, accurately weighing out the solute, dissolving the solute in the solvent, allowing the solution to reach equilibrium, filtering the solution, and properly storing the solution. Proper preparation of standard solutions is essential for accurate and reliable results in a variety of industries.