Ginni Rometty is an American business executive who is currently the executive chairman of IBM. She is known for her work in the field of technology and has played a key role in shaping the direction of the company during her tenure as CEO. According to Celebrity Net Worth, Rometty has an estimated net worth of $60 million.
Rometty began her career at IBM in 1981 as a systems engineer and has worked her way up through the ranks to become one of the most respected executives in the industry. She has held a number of leadership positions within the company, including head of sales and distribution, and was named president and CEO in 2012.
During her time as CEO, Rometty has focused on transforming IBM into a more innovative and agile company, with a particular emphasis on cloud computing and artificial intelligence. Under her leadership, IBM has made significant investments in these areas and has seen its stock price rise significantly.
In addition to her work at IBM, Rometty is also known for her philanthropic efforts. She has served on the board of directors for several non-profit organizations, including the National Academy Foundation and the U.S. Fund for UNICEF.
Rometty's success and leadership in the tech industry have earned her numerous accolades and awards, including being named one of Fortune's Most Powerful Women in Business for nine consecutive years and receiving the Edison Achievement Award for her contributions to the field of technology.
Overall, Ginni Rometty's net worth of $60 million is a reflection of her hard work and dedication to her career in technology. Her leadership and vision have helped to shape the direction of IBM and have made her one of the most respected executives in the industry.
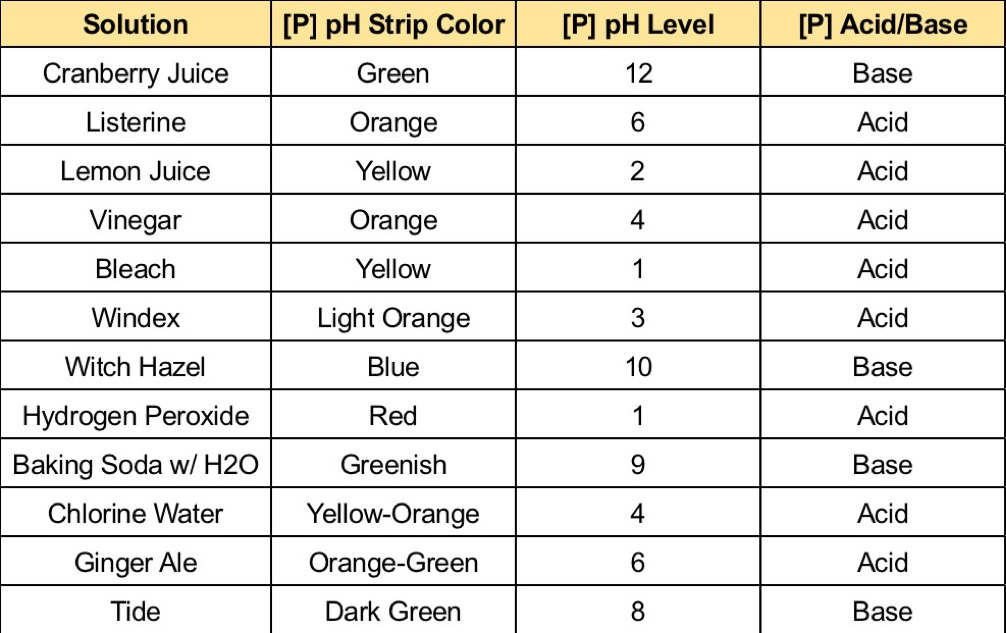
Is hydrogen peroxide a base or acid or neutral?
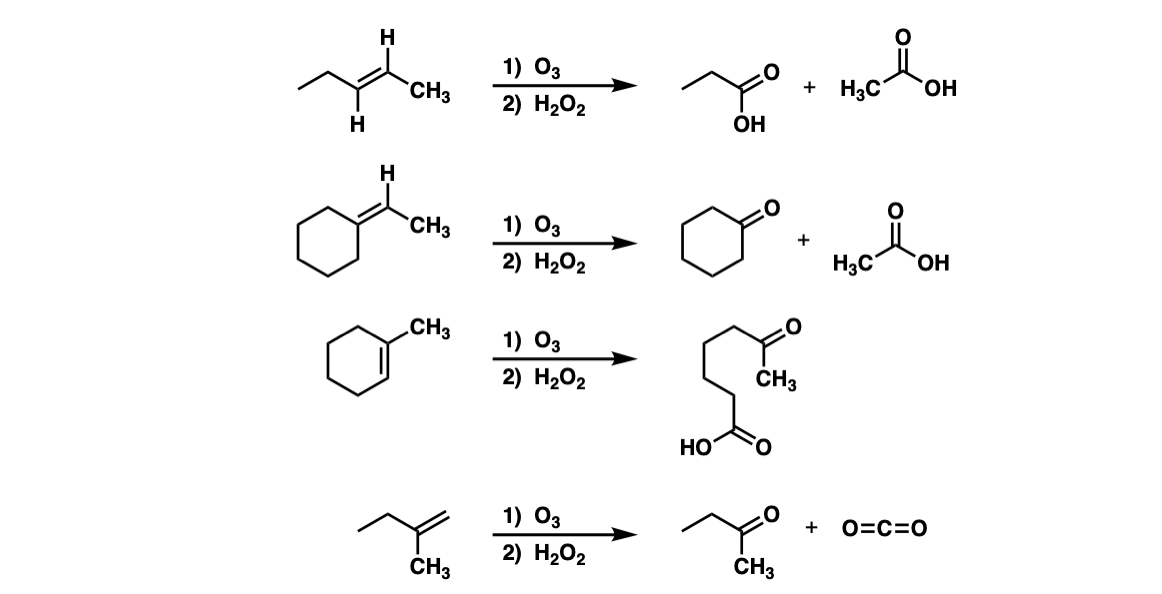
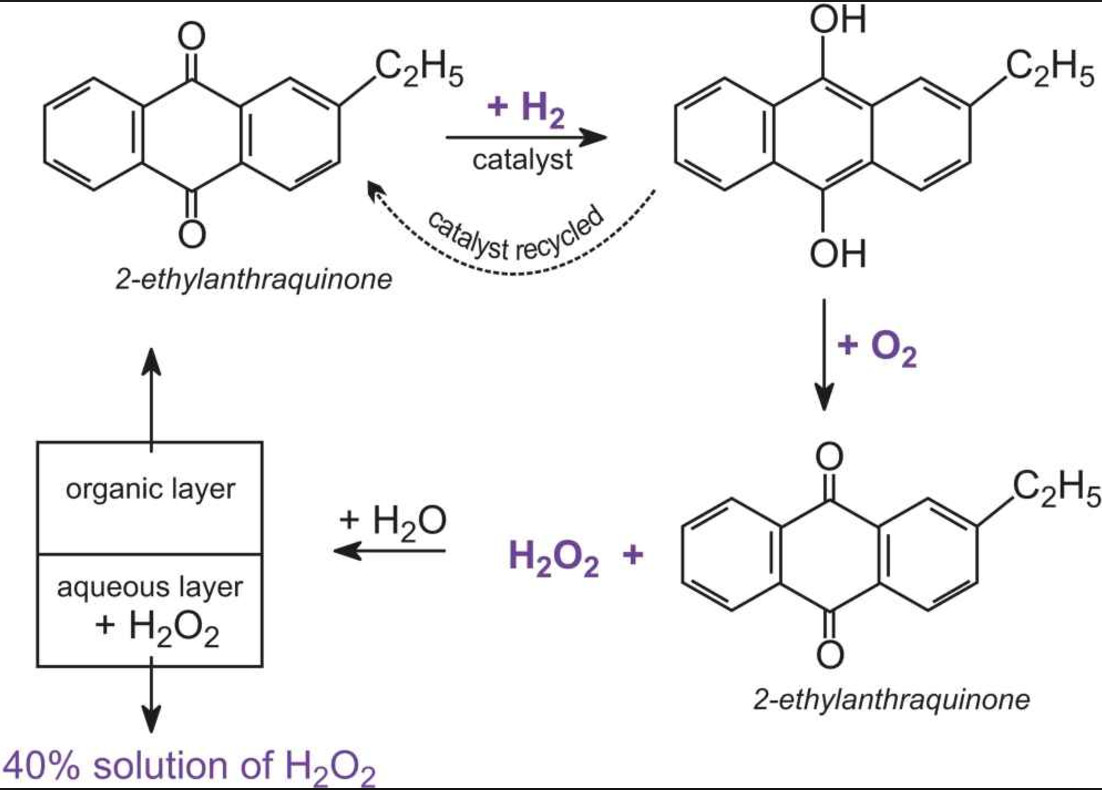
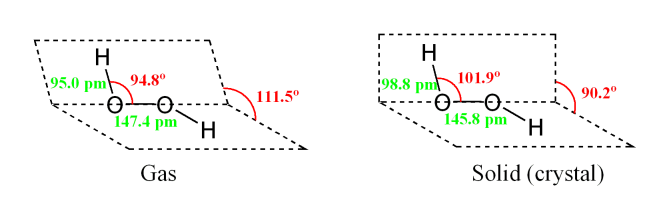
I am trying to prove that the more acidic or the more basic the reaction is, the more accelerated the reaction will be. Among the materials that you need to conduct this experiment are five clear containers, a washable spoon, distilled water, a measuring cup, baking soda, lemon juice, and a set of measuring spoons. In the aqueous solution the dissociation equation for hydrogen peroxide is given as: H2O2+H2OH3O++HO2- Hydroperoxide ion HO2-+H2OH3O++ O2 2- Peroxide ion The double arrow in reaction indicates a reversible reaction indicating that the ions formed after dissociation of hydrogen peroxide tend to reform the original molecules, hence, confirming that H2O2 is a weak acid. Dotted lines separate solid—liquid phases from solid—solid phases. There are several different types of chemical reactions and changes happening around us in our everyday lives.
Can hydrogen peroxide act as a Lewis acid and a Lewis base? : chemistry

Even though it is a weak acid, hydrogen peroxide is definitely This makes hydrogen peroxide more acidic than water naturally, meaning it should not be ingested or substituted for water under any conditions. See: Kingzett T 29 September 1882. Bases on the other hand, are usually of a pH higher than seven and are the opposite of acidic substances. The main reasoning behind this investigation is to discover how well the catalase enzyme in yeast can break down hydrogen peroxide after different amounts of acids and bases have been added onto it. Removal of blood stains Hydrogen peroxide reacts with blood as a bleaching agent, and so if a blood stain is fresh, or not too old, liberal application of hydrogen peroxide, if necessary in more than single application, will bleach the stain fully out.