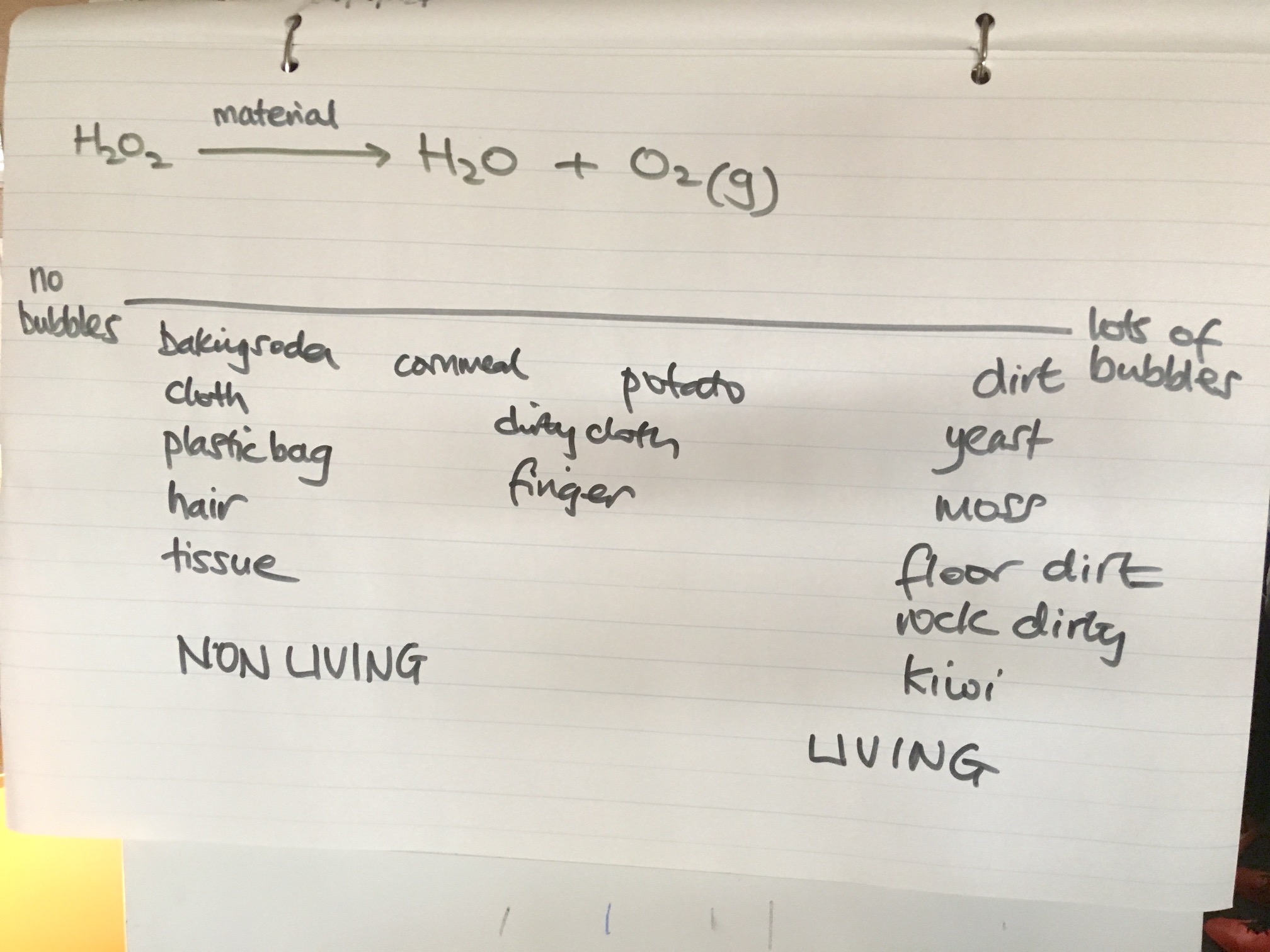
Hydrogen peroxide is a chemical compound with the formula H2O2. It is a pale blue liquid that appears colorless in a dilute solution. It is a powerful oxidizer and is used in a variety of applications, including as a disinfectant, a bleaching agent, and as a rocket propellant.
The potato, on the other hand, is a starchy root vegetable that is native to South America and is now cultivated around the world. Potatoes are a staple food in many countries and are used in a variety of dishes, such as mashed potatoes, roasted potatoes, and potato chips.
So what do hydrogen peroxide and potatoes have in common? One possible connection is that hydrogen peroxide can be used to bleach potatoes, as well as other vegetables, to make them appear more attractive. This is typically done by soaking the potatoes in a solution of hydrogen peroxide and water for a few minutes before cooking them. The hydrogen peroxide helps to remove any discoloration or blemishes on the surface of the potatoes, resulting in a more visually appealing final product.
Hydrogen peroxide can also be used as a natural disinfectant to kill bacteria and other harmful microorganisms on the surface of potatoes. This can be particularly useful when preparing potatoes for dishes that will be served raw, such as potato salads. Simply soaking the potatoes in a solution of hydrogen peroxide and water for a few minutes can help to kill any harmful bacteria and reduce the risk of foodborne illness.
In addition to its use in the food industry, hydrogen peroxide has a number of other applications. For example, it is often used as a hair bleach to lighten the color of hair, and it is also used in the production of certain types of paper and textiles. It has even been used as a rocket propellant, although this use is relatively rare.
In conclusion, hydrogen peroxide and potatoes may not seem to have much in common at first glance, but they can be connected through the use of hydrogen peroxide as a bleaching agent and disinfectant in the food industry. While hydrogen peroxide has a variety of other uses, its ability to improve the appearance and safety of potatoes is certainly an important one.