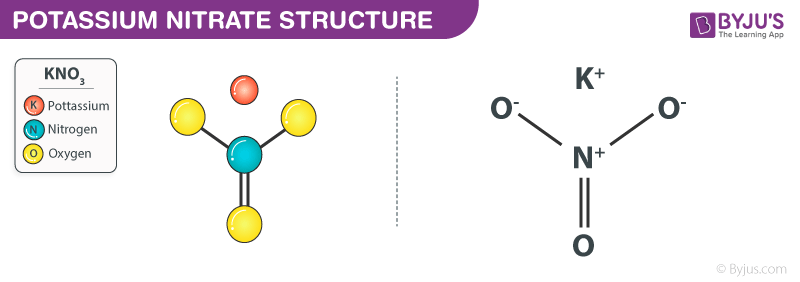
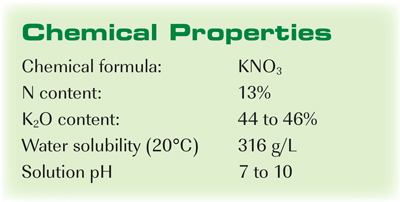
Potassium nitrate, also known as saltpeter, is a chemical compound with the formula KNO3. It is an ionic compound composed of potassium ions (K+) and nitrate ions (NO3-). Potassium nitrate is a white crystalline solid that is highly soluble in water, with a solubility of 36.2 g per 100 mL of water at 25 °C.
The solubility of a substance in water is a measure of how much of that substance can be dissolved in water at a given temperature. The solubility of potassium nitrate in water is relatively high due to the presence of both ionic bonds and hydrogen bonds between the potassium ions, nitrate ions, and water molecules.
Ionic compounds, such as potassium nitrate, are highly soluble in water because the water molecules are able to surround and solvate the ions, allowing them to move freely in solution. In the case of potassium nitrate, the positively charged potassium ions are attracted to the negative ends of the water molecules, while the negatively charged nitrate ions are attracted to the positive ends. This interaction results in the formation of a stable solution.
In addition to the solvating effect of the water molecules, hydrogen bonds also play a role in the solubility of potassium nitrate in water. Hydrogen bonds are a type of weak chemical bond that forms between a hydrogen atom and a highly electronegative atom, such as oxygen or nitrogen. In the case of potassium nitrate, the hydrogen atoms of the water molecules can form hydrogen bonds with the oxygen atoms of the nitrate ions. This interaction helps to stabilize the solution and increases the solubility of potassium nitrate in water.
In conclusion, potassium nitrate is highly soluble in water due to the presence of both ionic and hydrogen bonds between the potassium ions, nitrate ions, and water molecules. This solubility makes potassium nitrate a useful compound in various industrial and scientific applications, including the production of fertilizers, explosives, and glass.