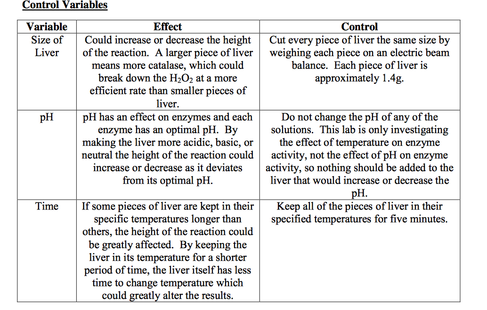
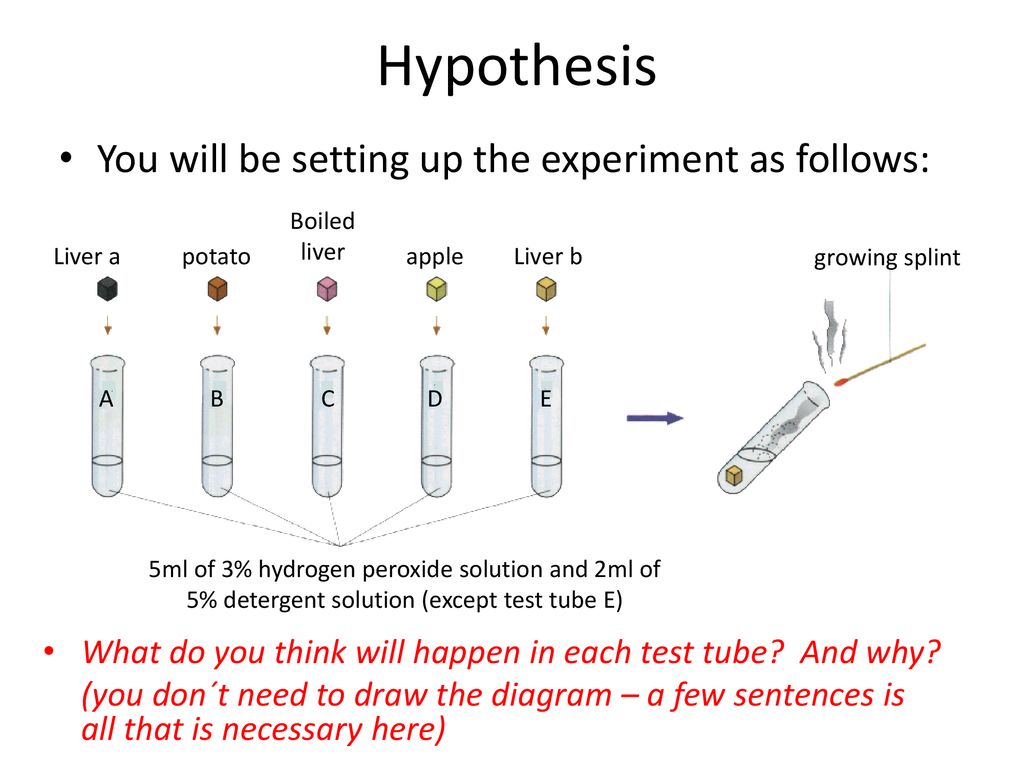
The liver is a vital organ in the human body that performs many important functions, including the detoxification of harmful substances and the synthesis of proteins and hormones. In a liver experiment with hydrogen peroxide, researchers might be interested in studying the effects of this substance on the liver and its ability to detoxify and protect the body.
Hydrogen peroxide is a chemical compound with the formula H2O2. It is a strong oxidizing agent that can be toxic to living cells, and it is commonly used as a disinfectant and bleaching agent. However, hydrogen peroxide can also have beneficial effects, as it can stimulate the production of certain enzymes that have antioxidant and anti-inflammatory properties.
In a liver experiment with hydrogen peroxide, researchers might use animal models, such as mice or rats, to study the effects of this substance on the liver. The animals would be given a specific dose of hydrogen peroxide, and the researchers would then observe the changes in liver function and tissue structure. They might also measure biomarkers in the blood or urine to assess the health of the liver and other organs.
One potential application of a liver experiment with hydrogen peroxide is to study the role of this substance in the detoxification process. The liver plays a critical role in the detoxification of harmful substances, and it can do this through various pathways, including the cytochrome P450 system, which is involved in the metabolism of drugs and toxins. In a liver experiment with hydrogen peroxide, researchers might investigate how this substance affects the activity of the cytochrome P450 system and other detoxification pathways in the liver.
Another potential application of a liver experiment with hydrogen peroxide is to study its effects on inflammation and oxidative stress. Inflammation and oxidative stress are important contributing factors to many diseases, including liver diseases. In a liver experiment with hydrogen peroxide, researchers might investigate whether this substance has any anti-inflammatory or antioxidant effects that could potentially be beneficial in the treatment of liver diseases.
Overall, a liver experiment with hydrogen peroxide can provide valuable insights into the effects of this substance on the liver and its role in the detoxification and protection of the body. Such research may have implications for the development of new treatments for liver diseases and other conditions.
The liver is a vital organ in the human body that is responsible for a number of functions, including the metabolism of nutrients, the production of bile, and the detoxification of harmful substances. In a recent experiment, researchers examined the effects of hydrogen peroxide on the liver to better understand the potential therapeutic uses of this compound.
Hydrogen peroxide (H2O2) is a chemical compound that is commonly used as a disinfectant and oxidizing agent. It is composed of two hydrogen atoms and two oxygen atoms, and it is known for its ability to break down into water and oxygen when exposed to light or heat. This characteristic makes it a useful tool for sterilizing surfaces and removing stains.
In the liver experiment, researchers exposed liver cells to various concentrations of hydrogen peroxide and observed the effects on the cells. They found that at low concentrations, hydrogen peroxide had a protective effect on the liver cells, helping to prevent oxidative stress and cell death. However, at higher concentrations, hydrogen peroxide was toxic to the liver cells and caused significant damage.
The researchers also examined the effects of hydrogen peroxide on liver function in animal models. They found that low doses of hydrogen peroxide improved liver function, while high doses caused liver damage. These findings suggest that hydrogen peroxide may have therapeutic potential for certain liver conditions, but more research is needed to determine the optimal dosage and duration of treatment.
One potential use for hydrogen peroxide in the liver is in the treatment of liver fibrosis, a condition in which scar tissue builds up in the liver and impairs its ability to function. Some studies have shown that hydrogen peroxide can help to reduce liver fibrosis by inhibiting the activation of certain proteins that contribute to the development of scar tissue.
However, the use of hydrogen peroxide as a therapeutic agent is not without risks. It can cause side effects such as nausea, vomiting, and skin irritation, and it can be toxic if ingested in large amounts. Therefore, it is important for researchers to carefully consider the potential benefits and risks of using hydrogen peroxide in the treatment of liver conditions.
In conclusion, the liver experiment with hydrogen peroxide showed that this chemical compound has both protective and toxic effects on the liver, depending on the concentration and duration of exposure. While it may have therapeutic potential for certain liver conditions, further research is needed to determine the optimal dosage and duration of treatment.