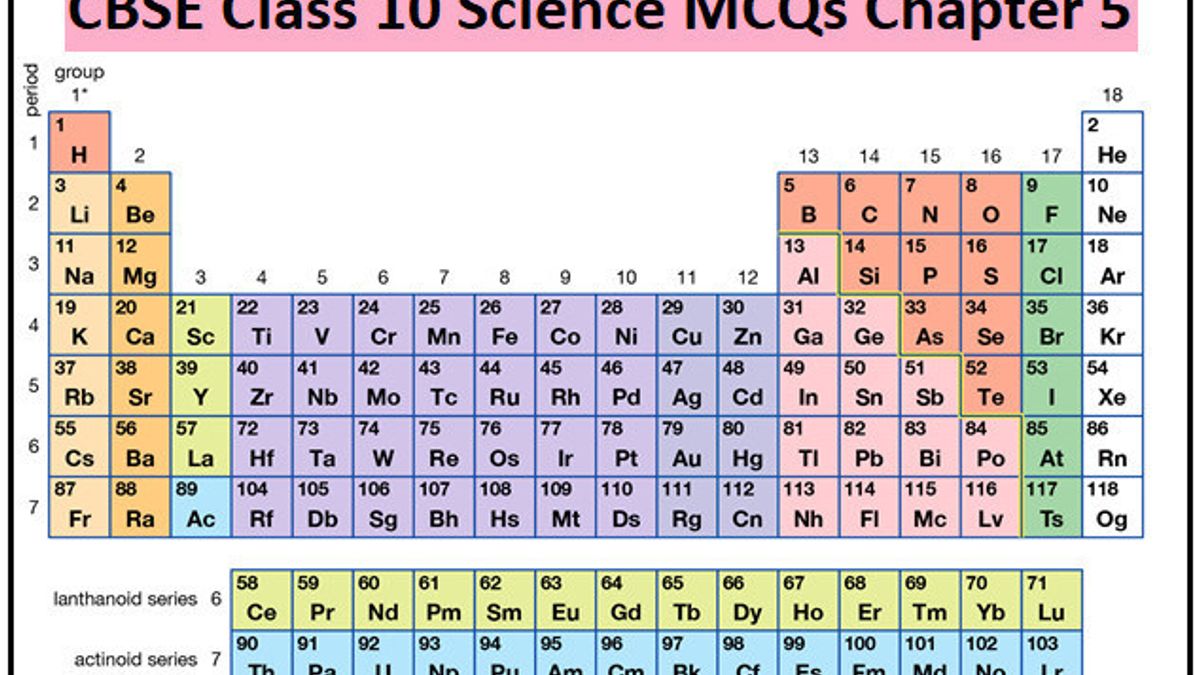
The modern form of the periodic table is a chart that arranges the chemical elements according to their atomic number, electron configurations, and recurring chemical properties. It was developed by Dmitri Mendeleev in the mid-19th century, and has since become the standard way to organize and classify the elements.
The periodic table is organized in rows and columns, with each element occupying a specific spot based on its atomic number. The atomic number is the number of protons in an atom's nucleus, and it determines the element's identity. Elements with similar properties are placed in the same column, known as a "period." For example, the alkali metals (lithium, sodium, potassium, etc.) are all placed in the first column of the periodic table because they have similar properties, such as being highly reactive and having a single valence electron.
The rows of the periodic table are called "periods," and each period corresponds to a specific electron shell. The first period consists of the elements with one electron shell, the second period has two electron shells, and so on. As you move down the periodic table, the atomic radius increases and the ionization energy decreases. This means that the elements at the bottom of the periodic table are generally more reactive and have lower melting and boiling points than the elements at the top.
The modern periodic table also includes several blocks of elements. The s-block elements are the alkali metals and alkaline earth metals, and they are characterized by having valence electrons in the s-orbital. The p-block elements are the nonmetals and metalloids, and they have valence electrons in the p-orbital. The d-block elements are the transition metals, and they have valence electrons in the d-orbital. Finally, the f-block elements are the lanthanides and actinides, and they have valence electrons in the f-orbital.
In addition to organizing the elements according to their properties, the periodic table also has several other important uses. It can be used to predict the properties of new elements that have not yet been discovered, and it can also be used to predict the behavior of elements in chemical reactions. It is an essential tool for chemists, and it is used in a wide range of fields, from biology and medicine to materials science and engineering.
Overall, the modern form of the periodic table is a vital tool for understanding and organizing the chemical elements. It has helped to revolutionize our understanding of the fundamental building blocks of matter and has played a crucial role in the development of many important technologies.