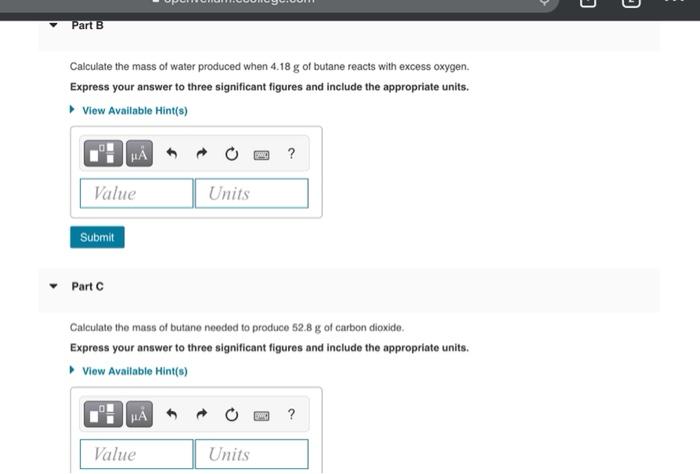
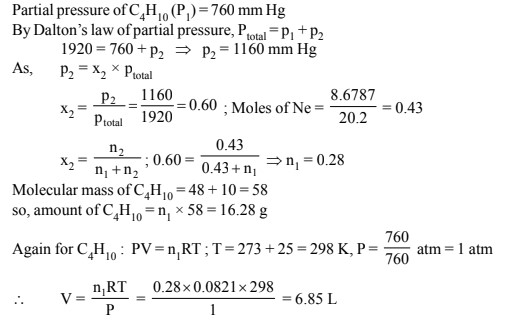
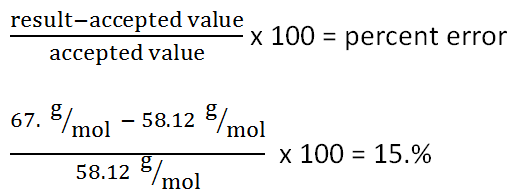The molar mass of a substance is the mass of one mole of that substance, and it is typically measured in grams per mole. Molar mass is an important concept in chemistry because it allows us to convert between the mass of a substance and the number of moles of that substance. In this essay, we will discuss the molar mass of butane, a hydrocarbon with the chemical formula C4H10.
Butane is a colorless, flammable gas that is commonly used as a fuel for lighters and portable stoves. It is also used as a feedstock in the petrochemical industry to produce a variety of chemicals, such as plastics and solvents. Butane is composed of four carbon atoms and ten hydrogen atoms, as indicated by its chemical formula.
To calculate the molar mass of butane, we need to add up the atomic weights of all of the atoms in its chemical formula. Carbon has an atomic weight of 12.0107 grams per mole, while hydrogen has an atomic weight of 1.00794 grams per mole. Using these values, we can calculate the molar mass of butane as follows:
Molar mass of butane = (4 x 12.0107 g/mol) + (10 x 1.00794 g/mol) = 48.0428 g/mol + 10.0794 g/mol = 58.1222 g/mol
Thus, the molar mass of butane is 58.1222 grams per mole. This value is important because it allows us to convert between the mass of butane and the number of moles of butane. For example, if we have 50 grams of butane, we can use the molar mass to calculate that we have 0.86 moles of butane, using the following formula:
Number of moles = mass / molar mass = 50 g / 58.1222 g/mol = 0.86 moles
In conclusion, the molar mass of butane is 58.1222 grams per mole, and it is a useful value for converting between the mass and the number of moles of this hydrocarbon. Understanding the molar mass of butane and other substances is an important part of chemistry, as it allows us to perform chemical calculations and understand the behavior of these substances at the molecular level.