"The World Made Straight" is a novel by Ron Rash that tells the story of Leonard Shuler, a young man living in the Appalachian Mountains of North Carolina in the 1970s. The novel is set against the backdrop of the region's tumultuous history, including the Civil War, the timber and tobacco industries, and the ongoing struggles of the working class.
At the beginning of the novel, Leonard is a high school dropout who is struggling to find his place in the world. He is drawn to the illicit world of marijuana farming, and begins working for a local dealer named Carlton Toomey. Leonard is drawn to the easy money and the sense of belonging that the drug trade provides, but he also struggles with feelings of guilt and the fear of getting caught.
As Leonard becomes more involved in the drug trade, he is forced to confront the harsh realities of the world around him. He witnesses the brutality of the drug business and the corruption that pervades every level of society. He also begins to understand the deep-seated injustices that have shaped his community, including the exploitation of the working class and the ongoing effects of the Civil War.
Despite these challenges, Leonard is able to find hope and redemption through his relationships with the other characters in the novel. He forms close bonds with his mentor, a former Vietnam War veteran named Travis, and with a young woman named Maddy, who helps him see the world in a different light. With their help, Leonard is able to confront his own demons and begin to build a better life for himself.
Ultimately, "The World Made Straight" is a powerful and moving story about the struggle for identity and the search for meaning in a world that is often harsh and unforgiving. Through the experiences of Leonard and the other characters, the novel offers a poignant commentary on the human condition and the enduring resilience of the human spirit.
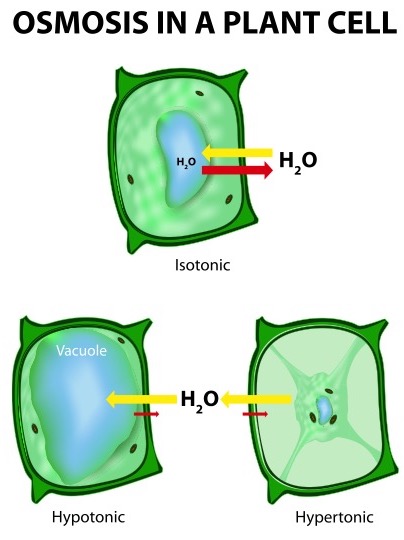
Osmosis is the movement of water molecules through a semi-permeable membrane from a region of high water concentration to a region of low water concentration. In plant cells, osmosis plays a vital role in regulating the concentration of solutes inside the cell and maintaining turgidity, or the state of being swollen or firm.
One common way to observe the effects of osmosis in plant cells is to conduct an experiment using a potato. To do this, a potato can be sliced into thin strips and placed in a variety of solutions with different solute concentrations. The solutions can range from pure water (high water concentration) to solutions with high concentrations of solutes, such as salt or sugar.
The results of the experiment can be observed by measuring the length and mass of the potato strips before and after they are placed in the solutions. If the potato strips are placed in a solution with a higher water concentration than the potato cells, the cells will absorb water through osmosis and become turgid. This will cause the potato strips to increase in length and mass. On the other hand, if the potato strips are placed in a solution with a lower water concentration than the potato cells, the cells will lose water through osmosis and become flaccid. This will cause the potato strips to decrease in length and mass.
In addition to measuring the length and mass of the potato strips, the experiment can also be observed by observing the change in shape of the potato cells under a microscope. If the cells are placed in a solution with a higher water concentration, the cells will become swollen and spherical in shape. If the cells are placed in a solution with a lower water concentration, the cells will become elongated and flattened in shape.
Overall, the results of the osmosis in plant cells experiment demonstrate the importance of osmosis in regulating the concentration of solutes inside plant cells and maintaining turgidity. This process is essential for the proper functioning and survival of plant cells, as it allows them to absorb and retain the water they need to grow and function properly.