P nitroacetanilide. Preparation of p 2023-01-06
P nitroacetanilide
Rating:
4,1/10
192
reviews
p-Nitroacetanilide, also known as p-nitroaniline, is a chemical compound with the molecular formula C6H7NO3. It is a pale yellow solid that is soluble in organic solvents such as ethanol, acetone, and ether, but not water.
p-Nitroacetanilide is used as a starting material in the synthesis of a variety of chemicals, including dyes, pharmaceuticals, and agrochemicals. It is also used as an intermediate in the production of other nitroaromatic compounds.
The synthesis of p-nitroacetanilide typically involves the nitration of acetanilide, which is done by reacting acetanilide with nitric acid and sulfuric acid. This reaction is known as the Sandmeyer reaction, named after the Swiss chemist Carl Julius von Sandmeyer who first described it in 1875. The yield of p-nitroacetanilide from this reaction can be improved by using a suitable catalyst, such as copper or iron.
p-Nitroacetanilide is highly toxic and can cause severe skin and eye irritation. It should be handled with caution and appropriate protective measures should be taken when working with it. In addition, p-nitroacetanilide is a potential carcinogen, and long-term exposure to it can increase the risk of cancer.
Despite its potential hazards, p-nitroacetanilide is an important chemical compound with a wide range of uses. It continues to be used in the synthesis of a variety of chemicals, including dyes, pharmaceuticals, and agrochemicals, and will likely remain an important chemical in the future.
What is the formula for p

And Ortho acetanilide remains to infiltrate due to its solubility in water. Crystallisation is carried out to break the solid-liquid form of p-Nitroacetanilide. Introduction to Preparation of p-Nitroacetanilide Nitration is an important reaction that is used within the training of nitro compounds. The acetamido institution -NHCOCH 3 in acetanilide is ortho and para directing. Is 4 nitroacetanilide insoluble in water? Principle p-Nitroacetanilide can be prepared by acetanilide nitration.
Next
p
Then, keep the mixture in an ice-bath, and a clear solution is obtained. Principle of p-Nitroacetanilide The p-Nitroacetanilide is prepared via nitration of acetanilide. Maintain the temperature below 10°C during addition. Safety Let us look at the safety points of p-Nitroaniline. Apart from this, a p-Nitroacetanilide hydrolysis reaction is carried out, and it is synthesised to obtain the P-nitroaniline.
Next
P

From all the above, we learned that p-Nitroacetanilide is known as Para Nitroacetanilide, prepared after the nitration of acetanilide. Also, when crystallised from ethyl alcohol, the p-nitro acetanilide crystallises mostly as colourless crystals, whereas, the ortho isomer remains in the same solution. At AB Enterprises, we offer these quality chemical substances and compounds to companies in the sector of Pharmaceuticals manufacturing, Textile Industries, Plastic Dyeing , Food Colouring Industries, Paint Manufacturing and Chemical Refineries across markets to several destinations including India and Indian Subcontinent, East Asia, South East Asia, United States of America and other European countries. Owing to this, the Para Nitroacetanilide gets easily crystallised. Moreover, it is used to develop poultry medicines, anti-oxidants, gasoline, pesticides, and rubber chemicals. However, during crystallisation, it gets isolated in a flask while the P-Nitroacetanilide takes the shape of crystals and are separated with filter paper. Filter the hot solution and cool the filtrate.
Next
4'
Allow it to stand for 15 minutes. It was also used to prepare 4-aminoacetanilide. Basic Experiments The names and gram molecular weights of main compounds involved in this is Chemistry experiment are listed below. Lastly, p-Nitroacetanilide is also widely used in gum and corrosion inhibitors. However, o-Nitroacetanilide is a readily soluble substance. It is advised to avoid direct or indirect contact and does not inhale or ingest.
Next
Experiment

Uses Manufacture of nitraniline. Lastly, the acetanilide is purified via the process of crystallisation. What is the Colour of minor product formed in the preparation of p-nitroacetanilide? Moreover, the compound is often known as 4-Nitroacetanilide, P-Acetamido Nitrobenzene, N- 4-nitrophenyl acetamide and N-Acetyl-4-nitroaniline. Hence, it is named Para Nitroacetanilide. Depending on the preparation, the colour can also be brown-green or green-yellow.
Next
Preparation of p

The compound has a phenyl group that is connected to an amino group and is para adjacent to a nitro group. However, the primary purpose of preparing the p-Nitroacetanilide is to obtain the p-Nitroaniline. Uses of p-Nitroacetanilide As discussed earlier, p-Nitroacetanilide has analgesic properties which is helpful to the pharmaceutical industry to prepare several medications, like paracetamol. It appears yellow solid. Therefore, on nitration a mixture of o- and p-nitroacetanilide is formed: The acetamido group being a bulky group causes steric hindrance at the ortho position. Before moving further, here is a summary of acetanilide. H2SO4 in a beaker.
Next
Nitration is described as an important reaction that is used in the nitro compound preparation. Additionally, ions of acetic acid are poor nucleophiles, which ensure that there will be no substitution reaction during dissolving. Nitroacetanilide is a combination of Nitro and Acetanilide. How can we separate p-nitroacetanilide from O-nitroacetanilide in the crude sample? The Image will be Uploaded Soon It is a type of electrophilic substitution reaction. It can be used to manufacture dyes, medicines, anti-oxidants, and gasoline, which have become essential in current times. Acetanilide has no odour.
Next

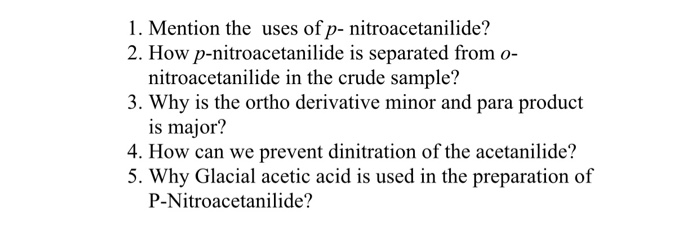
Conclusion As we came so far, we learned all the aspects of p-Nitroacetanilide. An organic substance, acetanilide, is processed through nitration to prepare p-Nitroacetanilide. Hydroxylamine hydrochloride NH 2 OH. On crystallisation fiom ethyl alcohol, p-nitroacetanilide crystallises out as almost colourless crystals while the ortho isomer remains in solution. On crystallization from ethyl alcohol, p-nitroacetanilide crystallizes out as nearly colorless crystals even as the ortho isomer stays in solution.
Next

Synthesis Reference s Journal of the American Chemical Society, 75, p. Therefore, the p-nitroacetanilide is formed as the major product. However, excessive consumption or exposure can lead to respiratory and skin problems. After this, a purification process is processed to obtain pure P-Nitroacetanilide. In this experiment, you will preparep-nitroacetanilide by the nitration of acetanilide. Now, dry the crystals in filter paper folds and weigh them to know the yield.
Next

HCl is defined as corrosive; hence, avoid all contact and handle this compound with caution. Pour the mixture on to crushed ice 20 g with stirring. The acetarnido group -NHCOCH3 in acetanilide is ortho and para directing. It is an organic substance produced by acetic anhydride with aniline. Further, the separation of impurities or unnecessary components is the main reason behind crystallisation processed on nitrified acetanilide. As a result, purified p-Nitroacetanilide is obtained, and the melting point is also determined. Filter, dry and record the melting point and TLC using toluene as solvent.
Next








