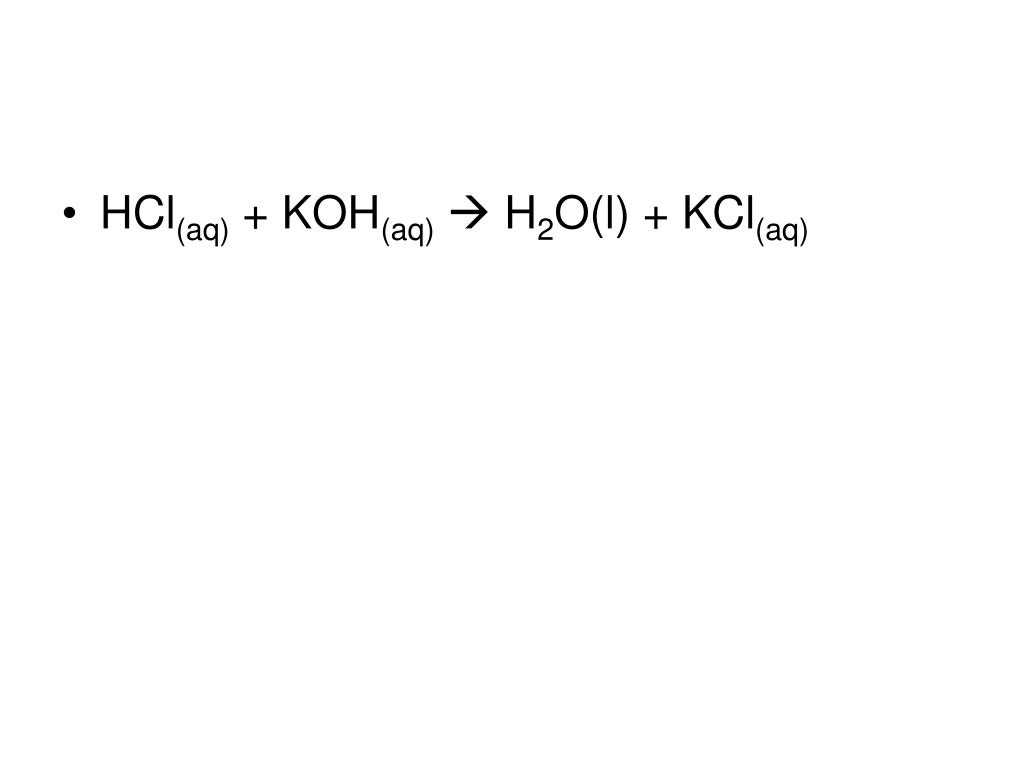Potassium hydroxide and hydrochloric acid are both common chemicals with a variety of uses in industry, research, and household applications. Both substances have their own unique properties and reactivity, and they can be used in combination to produce useful chemical reactions.
Potassium hydroxide, also known as caustic potash, is a strong base with the chemical formula KOH. It is a white, crystalline solid that is highly soluble in water, and it has a strong alkaline pH of around 14. Potassium hydroxide is often used in the production of soaps, detergents, and other cleaning agents due to its ability to effectively remove grease and oils. It is also used in the manufacturing of plastics, resins, and other synthetic materials.
Hydrochloric acid, also known as muriatic acid, is a strong acid with the chemical formula HCl. It is a clear, colorless liquid that has a pungent, corrosive smell, and it has a pH of around 0. Hydrochloric acid is commonly used in the production of fertilizers, dyes, and other chemicals. It is also used in the refining of metals and in the pickling of steel to remove rust and impurities.
When potassium hydroxide and hydrochloric acid are mixed together, they react to produce water and a salt called potassium chloride. The reaction between these two chemicals is known as a neutralization reaction, which occurs when an acid and a base react to form a salt and water. The general equation for this reaction is:
Acid + Base → Salt + Water
In the case of potassium hydroxide and hydrochloric acid, the reaction can be written as:
HCl + KOH → KCl + H2O
The reaction between potassium hydroxide and hydrochloric acid is an exothermic reaction, which means that it releases heat as it occurs. The heat produced during the reaction can be used to power other chemical reactions, or it can be used to generate electricity.
In conclusion, potassium hydroxide and hydrochloric acid are two important chemicals with a wide range of applications in industry and research. When mixed together, they react to form a salt and water, and the heat produced during this reaction can be harnessed for other purposes. Understanding the properties and reactivity of these chemicals is important for their safe handling and use in various applications.