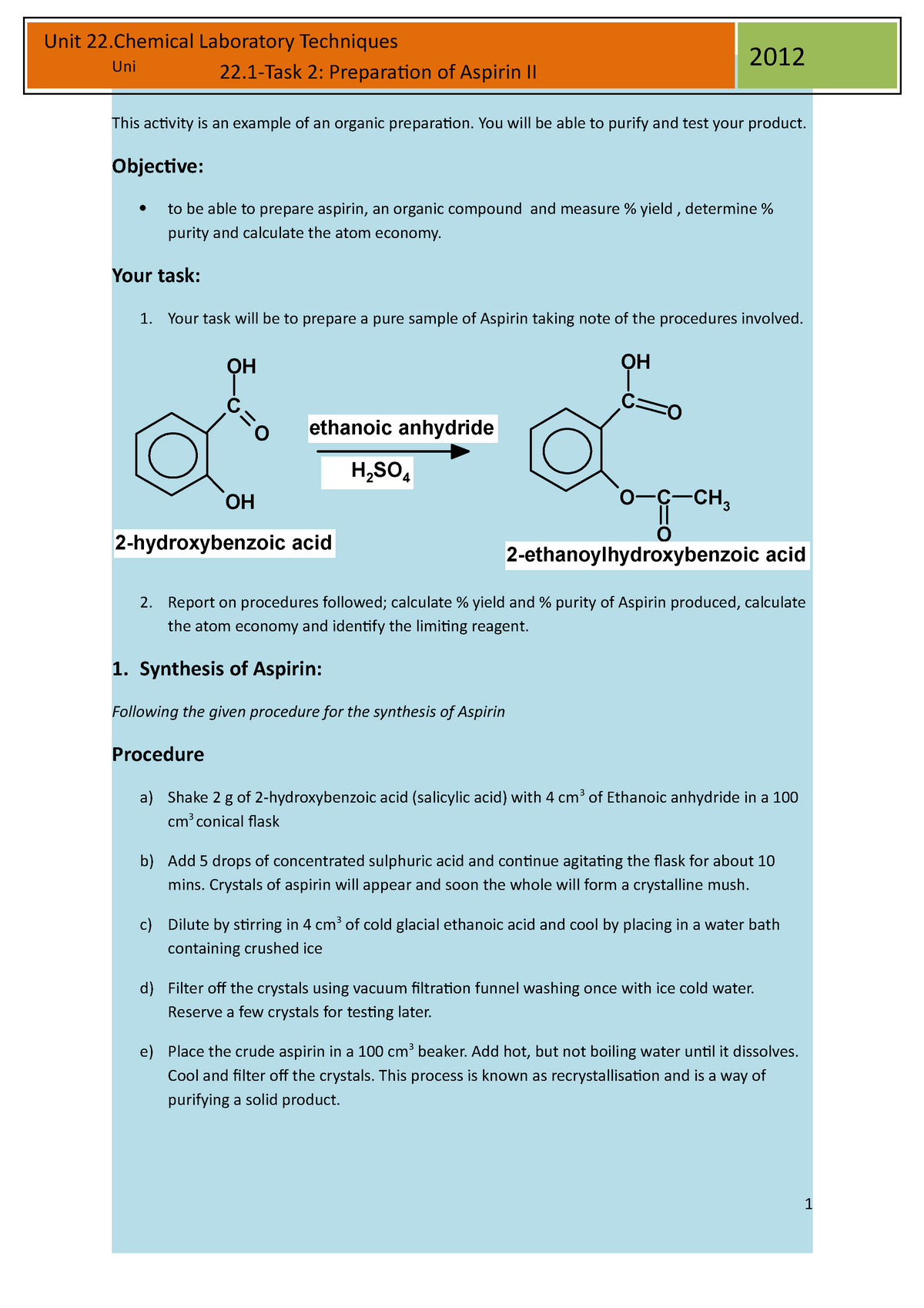
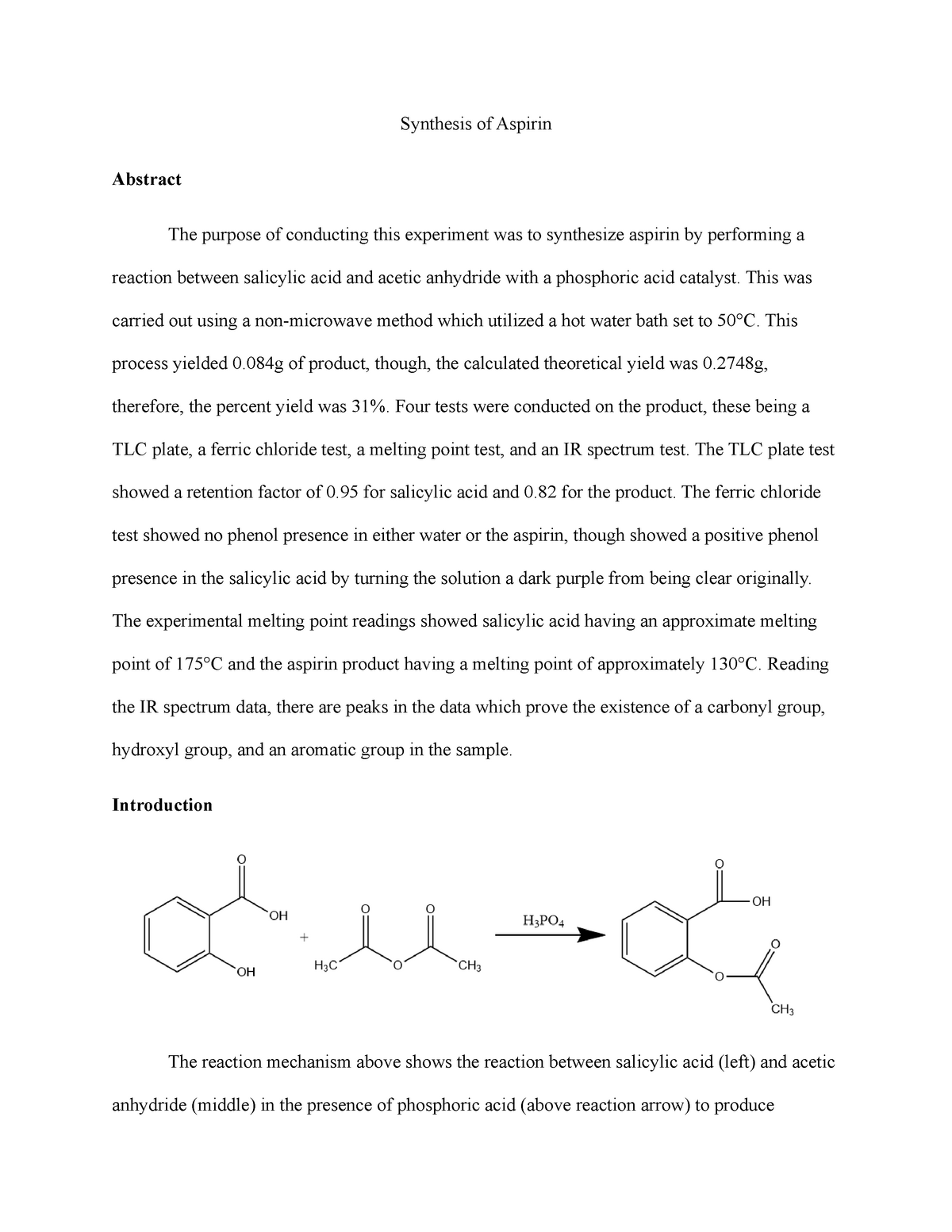
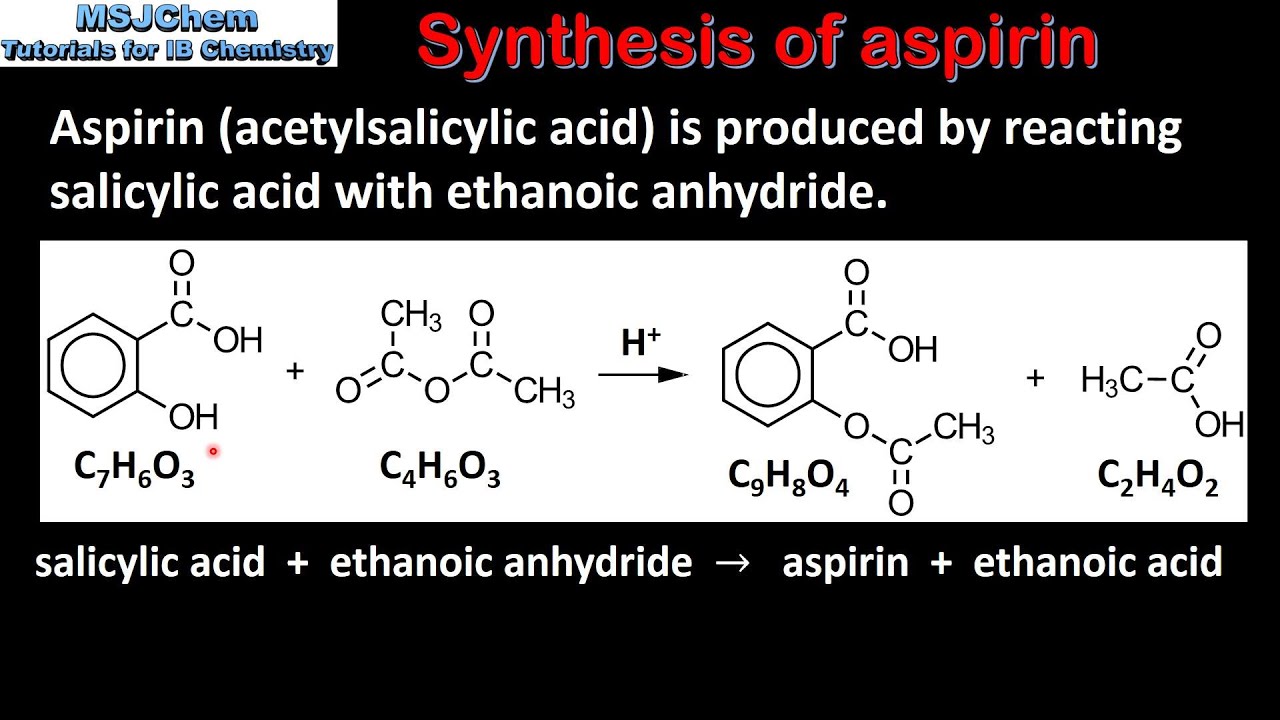
Aspirin, also known as acetylsalicylic acid, is a common pain reliever and fever reducer. It is widely used in both over-the-counter and prescription medications. The preparation of aspirin involves a chemical reaction called esterification, which involves the reaction of an acid and an alcohol. In this experiment, salicylic acid will be used as the acid and methanol as the alcohol.
Before starting the experiment, it is important to gather all necessary materials and equipment. These may include:
- Salicylic acid
- Methanol
- Sulfuric acid
- Distilled water
- Magnetic stirrer
- Stir bar
- Stir plate
- 50 mL round bottom flask
- Reflux condenser
- Thermometer
- Vacuum filtration setup
- Vacuum pump
- Vacuum hose
- Vacuum filter flask
- Buchner funnel
- Vacuum filtration paper
- Vacuum filtration flask stand
- Vacuum filtration flask clamp
It is also important to wear appropriate personal protective equipment, such as goggles and lab coat, to ensure the safety of the experimenter.
To begin the experiment, the round bottom flask should be placed on the stir plate and the stir bar should be added to the flask. Next, add 5.0 grams of salicylic acid and 5.0 mL of methanol to the flask. The mixture should be stirred until the salicylic acid is fully dissolved.
Next, add 5 drops of sulfuric acid to the mixture and continue stirring. The sulfuric acid acts as a catalyst in the esterification reaction and helps to increase the reaction rate. The mixture should be heated to a temperature of around 70-80°C, using a heating mantle or hot water bath. It is important to monitor the temperature using the thermometer and to maintain the temperature within this range throughout the reaction.
Once the mixture has reached the desired temperature, the reflux condenser should be attached to the round bottom flask. The reflux condenser helps to prevent the loss of volatile reactants and products during the reaction. The reaction should be allowed to proceed for approximately 2-3 hours, or until the theoretical yield of aspirin is obtained.
After the reaction is complete, the mixture should be cooled to room temperature and then filtered using a vacuum filtration setup. To do this, the vacuum pump should be connected to the vacuum hose, which is then connected to the vacuum filter flask. The vacuum filter flask should be placed on the vacuum filtration flask stand and secured using the vacuum filtration flask clamp. The vacuum filtration paper should be placed on the Buchner funnel, which is then placed in the neck of the vacuum filter flask.
The mixture should be carefully poured onto the vacuum filtration paper, using a funnel if necessary. The vacuum pump should be turned on, which will create a vacuum in the vacuum filter flask. This will cause the mixture to be drawn through the vacuum filtration paper and into the vacuum filter flask. Once all of the mixture has been filtered, the vacuum pump should be turned off and the vacuum filtration setup should be disassembled.
The aspirin should be washed with a small amount of distilled water to remove any impurities, and then allowed to dry. The dried aspirin can be weighed and the yield calculated by comparing it to the theoretical yield.
In conclusion, the preparation of aspirin involves the chemical reaction of salicylic acid and methanol, catalyzed by sulfuric acid. Careful attention must be paid to the materials, equipment, and safety precautions used during the experiment. By following these steps,