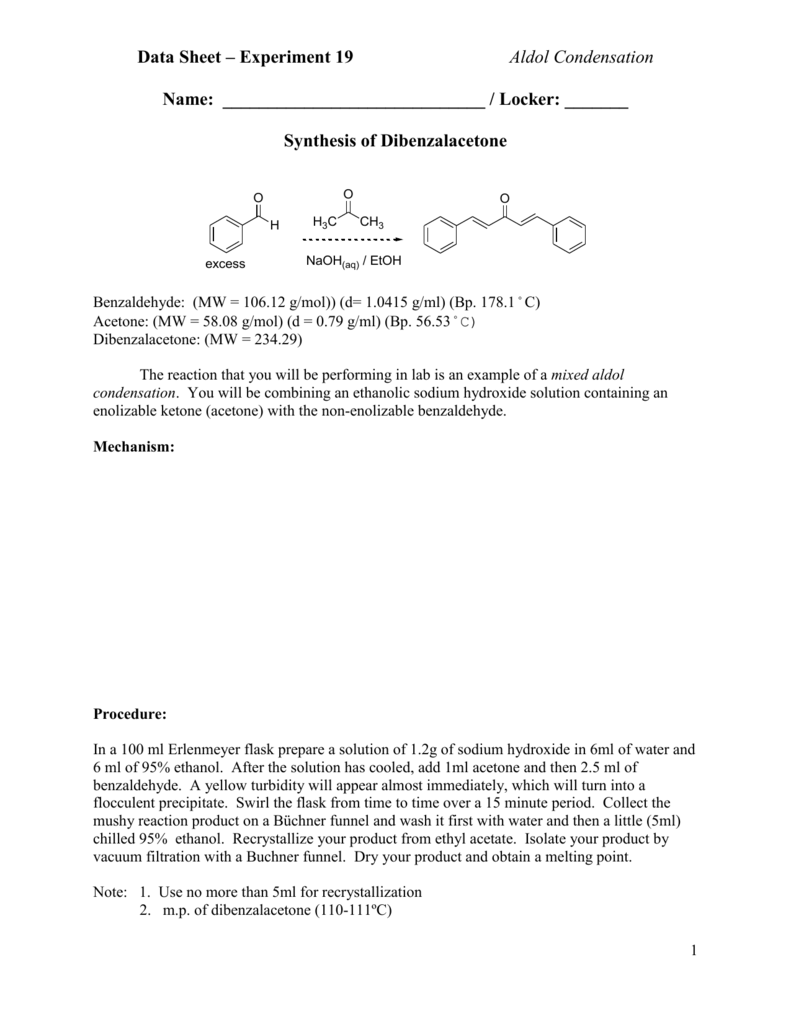
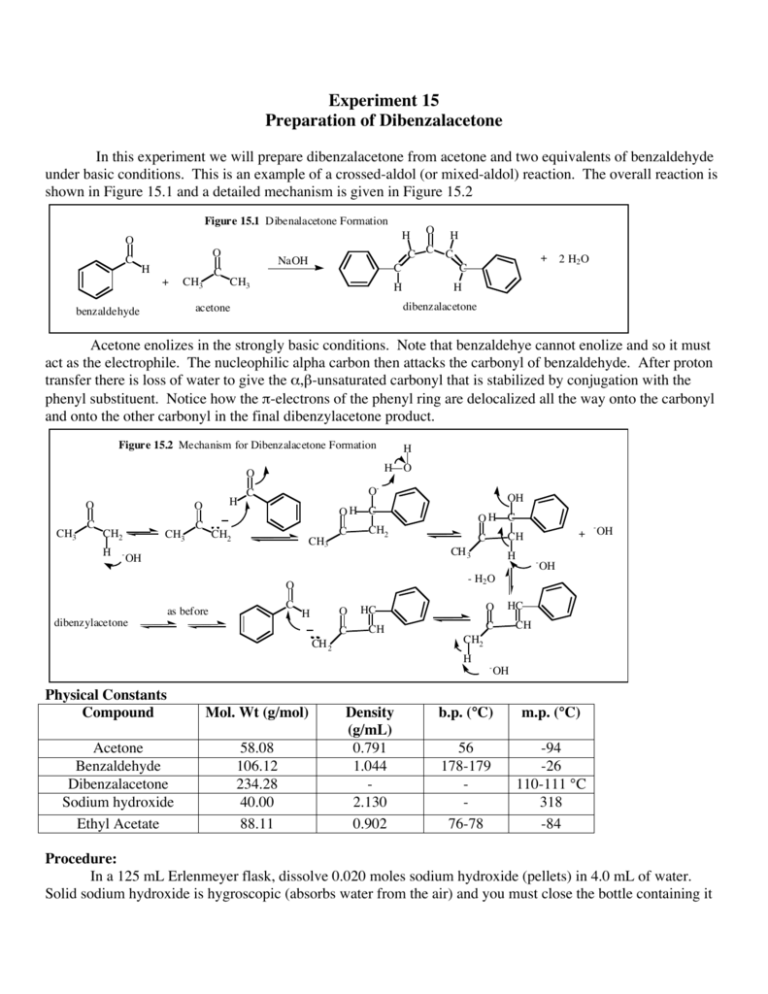
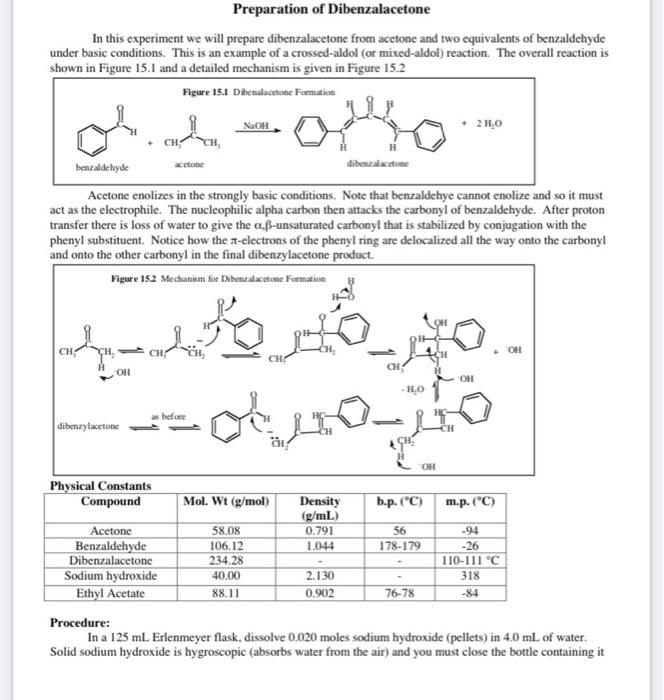
A dibenzalacetone lab report is a document that describes the results of a laboratory experiment in which dibenzalacetone is synthesized from benzaldehyde and acetone. This report should include a detailed description of the experimental procedure, the results obtained, and any observations made during the experiment. In addition, the report should include a discussion of the relevant theory and a conclusion that summarizes the main findings and implications of the experiment.
To prepare a dibenzalacetone lab report, the first step is to carefully read and understand the laboratory manual or guidelines provided by the instructor. This will give you an overview of the experiment and help you understand what is expected of you.
Next, you should carefully perform the experiment, following the instructions in the manual or guidelines. As you conduct the experiment, it is important to make detailed notes of the steps you take, the materials you use, and any observations you make. These notes will be very useful when it comes time to write your report, as they will help you accurately describe the experimental procedure and results.
Once the experiment is complete, you should analyze your results and interpret them in light of the relevant theory. This may involve performing calculations or making graphs to better understand the data. It is important to be thorough and accurate in your analysis, as this will be an important part of the report.
Finally, you should write the report itself. The report should be organized and clearly written, with each section following a logical structure. It should begin with an introduction that provides background information on dibenzalacetone and the purpose of the experiment. The next section should describe the experimental procedure in detail, including the materials used and the steps taken. The results section should present the data obtained during the experiment and any calculations or graphs that help to interpret the data. The discussion should interpret the results in light of the relevant theory and draw any conclusions that are supported by the data. The report should end with a conclusion that summarizes the main findings and implications of the experiment.
In summary, preparing a dibenzalacetone lab report requires careful planning, careful execution of the experiment, thorough analysis of the results, and clear and concise writing. By following these steps, you can produce a high-quality lab report that accurately and effectively communicates the results of your experiment.