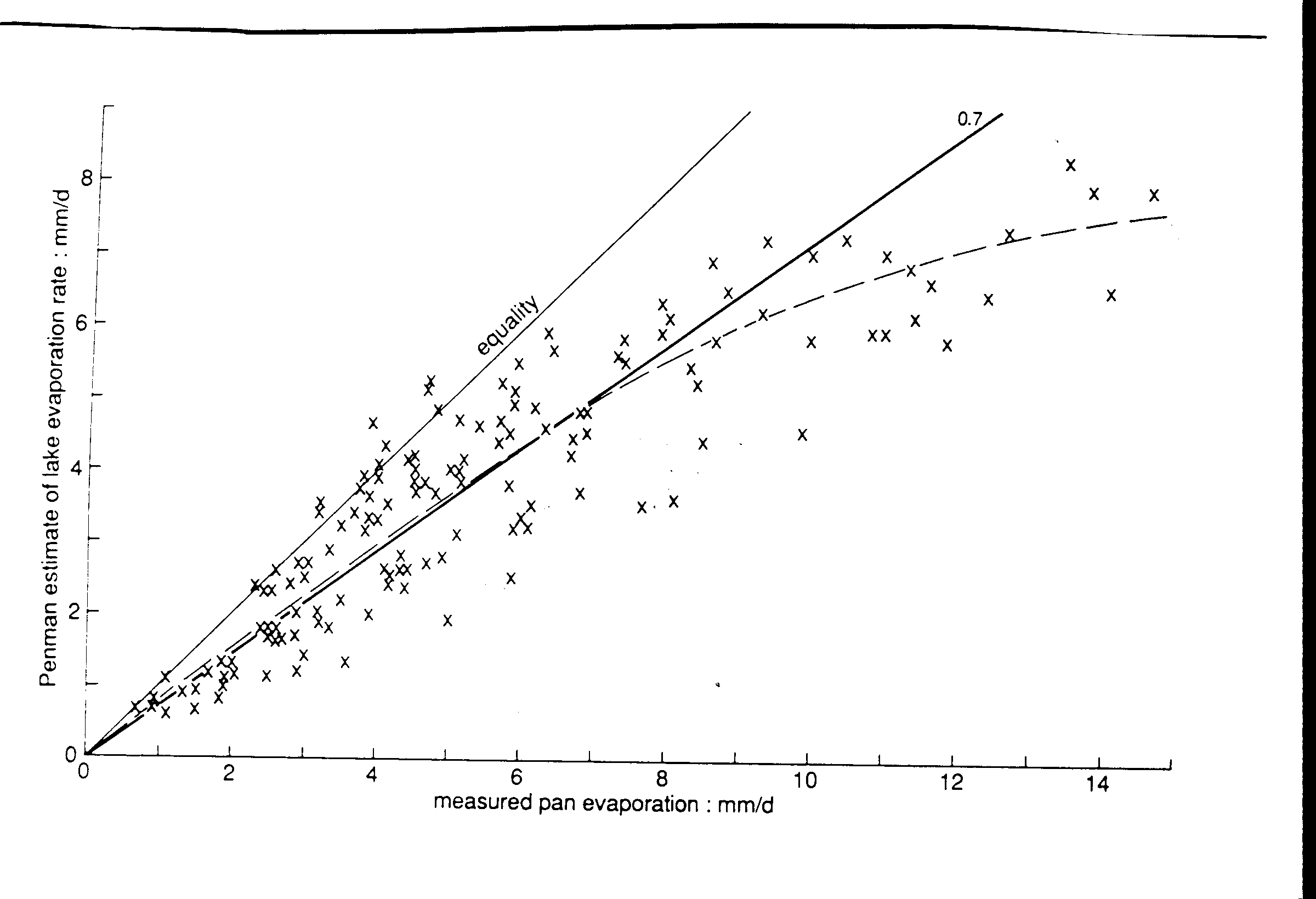
Evaporation is the process by which a liquid, such as water, turns into a gas. It is a fundamental part of the water cycle and plays a significant role in the regulation of Earth's temperature and humidity. The rate at which a liquid evaporates is influenced by several factors, which can include the temperature, humidity, and wind speed of the surrounding environment, as well as the nature of the liquid itself.
One of the most important factors that affects the rate of evaporation is temperature. As the temperature of a liquid increases, the molecules within it gain more energy and are more likely to escape from the liquid's surface and enter the air as a gas. This means that warmer liquids will generally evaporate more quickly than colder liquids.
Another factor that can affect the rate of evaporation is humidity. Humidity is a measure of the amount of water vapor present in the air. When the air is already saturated with water vapor, it is more difficult for additional vapor to escape from a liquid's surface and enter the air. This means that the rate of evaporation will be slower in humid environments compared to dry environments.
Wind speed is another factor that can influence the rate of evaporation. When a liquid is exposed to wind, the moving air molecules can help to carry away the evaporating molecules and increase the rate of evaporation. This is why you may notice that clothes dry more quickly on a clothesline on a windy day compared to a still day.
The nature of the liquid itself can also play a role in the rate of evaporation. Some liquids, such as alcohol, are more volatile and will evaporate more quickly than others, such as oil. The surface area of the liquid can also affect the rate of evaporation. A larger surface area means that there is more opportunity for molecules to escape into the air, which can increase the rate of evaporation.
In conclusion, the rate of evaporation is influenced by a number of factors, including temperature, humidity, wind speed, and the nature of the liquid. Understanding how these factors affect the rate of evaporation can be useful in a variety of applications, including the design of cooling systems and the preservation of perishable goods.