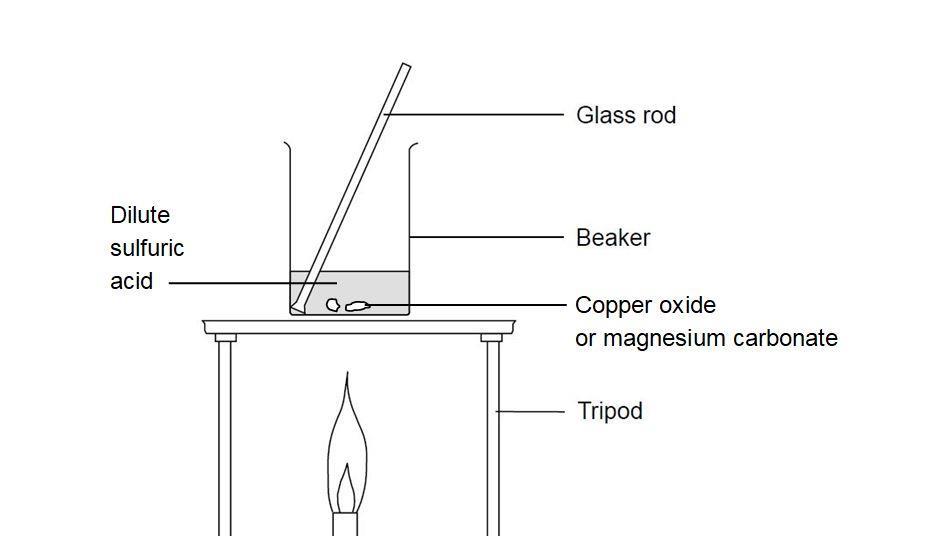
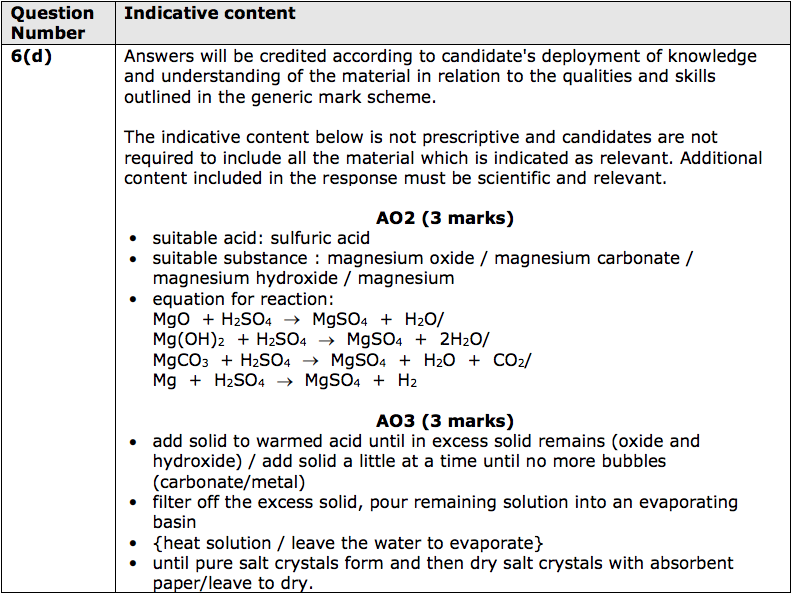
Magnesium oxide (MgO) is a compound composed of magnesium and oxygen. It is an ionic compound, meaning that it is composed of positive and negative ions that are held together by strong electrostatic forces. Magnesium oxide is a white solid that is commonly found in nature as the mineral periclase. It is also known as magnesia and is used in a variety of applications, including as a refractory material, a chemical feedstock, and a dietary supplement.
Hydrochloric acid (HCl) is a strong acid that is composed of hydrogen and chlorine. It is a corrosive, colorless liquid that has a pungent, strong smell. Hydrochloric acid is widely used in a variety of industries, including the production of fertilizers, dyes, and plastics.
When magnesium oxide is mixed with hydrochloric acid, a chemical reaction occurs that produces magnesium chloride (MgCl2) and water. The balanced chemical equation for this reaction is:
MgO + 2HCl → MgCl2 + H2O
In this reaction, the magnesium oxide and hydrochloric acid react to form magnesium chloride and water. The magnesium oxide and hydrochloric acid are the reactants, while the magnesium chloride and water are the products.
The reaction of magnesium oxide and hydrochloric acid is an example of a neutralization reaction. Neutralization reactions occur when an acid and a base react to form a salt and water. In this case, the magnesium oxide acts as a base, while the hydrochloric acid acts as an acid. The reaction of these two substances results in the formation of the salt (magnesium chloride) and water.
The reaction of magnesium oxide and hydrochloric acid is also an exothermic reaction, which means that it releases heat. This can be observed by the temperature increase that occurs during the reaction.
In conclusion, the reaction of magnesium oxide and hydrochloric acid is a neutralization reaction that produces magnesium chloride and water. This reaction is exothermic and releases heat. Magnesium oxide and hydrochloric acid are commonly used in a variety of industrial and scientific applications, and understanding the reaction between these two substances is important for the safe handling and use of these chemicals.


.JPG)