In a lab setting, the reaction between zinc and iodine can be observed by mixing a small amount of powdered zinc with iodine crystals. The resulting reaction is exothermic, meaning that it releases heat, and produces a yellow solid called zinc iodide.
To begin the experiment, it is important to gather all necessary materials and set up the lab space according to proper safety protocols. This includes wearing protective eyewear and gloves, as well as having a fire extinguisher on hand in case of any accidents.
Once the materials are ready, a small amount of powdered zinc is placed in a clean, dry test tube. A crystal or two of iodine is then added to the test tube, and the resulting reaction is observed. The iodine will immediately begin to sublimate, or turn from a solid directly into a gas, and the zinc will start to react with the iodine vapor, forming zinc iodide.
As the reaction progresses, the test tube may become warm to the touch due to the release of heat. The reaction will also produce a yellow solid, which is the zinc iodide that has formed. The reaction is typically complete within a few minutes, at which point the test tube can be removed from the heat source and allowed to cool.
The reaction between zinc and iodine can be further studied by measuring the amount of heat that is produced during the reaction. This can be done using a calorimeter, which is a device that is specifically designed to measure the heat of a chemical reaction. By measuring the heat produced during the reaction, scientists can learn more about the nature of the reaction and the amount of energy that is involved.
Overall, the reaction between zinc and iodine is a useful and interesting example of a chemical reaction that can be observed in the lab. It allows students to learn about the properties of different elements and how they interact with each other, as well as the fundamental principles of thermochemistry.
Determination of Chemical Formulae

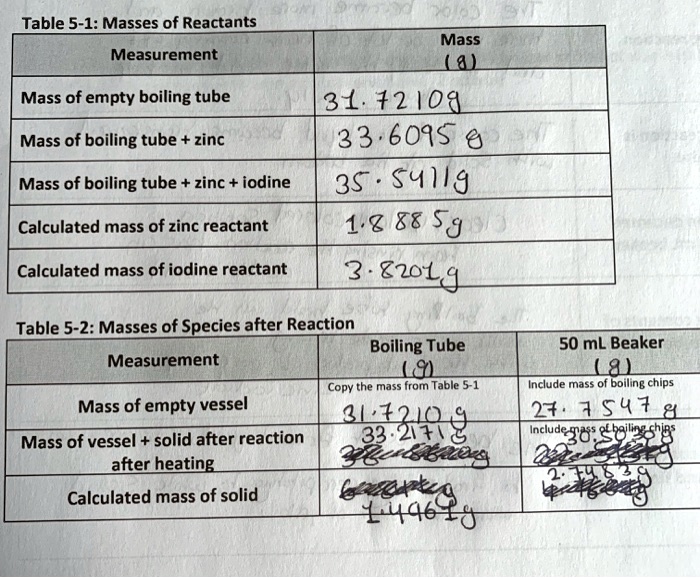
Suppose, for example, that the combustion experiment for a compound that contains carbon, hydrogen, and oxygen gives the compositions as 40. Method 1 To set up the reaction between zinc and iodine known as trial 1 , 2. I had to find the empirical formula for Zinc Iodide for a chem lab. Procedure To start the formula, the researchers weighted out approximately 2 grams of Zinc and Iodine. This solution is then decanted via the pouring of the solution to a 50 mL beaker with a boiling chip, weighed.
Determination of a Chemical Formulae lab write up: Experiment 1

Experimental Section Materials 20 to granular zinc metal solid iodine 6 M acetic acid distilled water glazed weighing paper desiccator plastic bags with silica gel desiccant copper wire electrodes 9 V battery electrode connectors 100 mL beaker 200 mL beaker 50 mL beaker 25 mL graduated cylinder spatula balance glass stirring rod heating plate Procedure The mass of a 100 mL beaker and a piece of glazed weighing paper was recorded 0 g. The law of This paper details the results of an experiment to produce zinc iodide from the reaction of zinc with iodine, making use of the laboratory setup given to investigate aspects of the reaction relating to the conservation of mass, the identification of the limiting reagent, and the observations on the physical characteristics of the solution as it underwent the reaction Spatafora, 2010; Nuffield Foundation, 2013. The E cell consists of an anode gains electrons , a cathode loses electrons , a salt bridge, and two ion solutions. For trial one, we had. To determine the percent yield of a synthesis reaction. Whenever dealing with percentages in a calculation of this type, one of the easiest ways to proceed is to assume that you have 100 g of the substance.
Lab: Preparation of Zinc Iodide

Review the MSDS for 2 and briefly summarize the hazards. The bulb should glow to show that the circuit is complete, and that electrolysis is occurring. Zinc iodide is a chemical compound of zinc and iodine, ZnI 2. The balanced chemical equation consists of stoichiometric factors which relate the number of moles of each species, not the mass ratios. For each cell potential voltage, a clean salt bridge was placed into the two metal solutions which connected the half cells. This law was proven by Antoine Lavoisier 1743 — 1794 , and stated that matter can go through any physical or chemical reaction, but the total mass of the reactants and the products will remain equal Rogers et al.