Standardization of sodium hydroxide, or NaOH, is the process of determining the concentration of a solution of NaOH. This is important because NaOH is a strong base, which means it can neutralize acids and has a high pH. It is used in a variety of industrial and laboratory settings, including in the production of soap, paper, and textiles, as well as in wastewater treatment and chemical analysis.
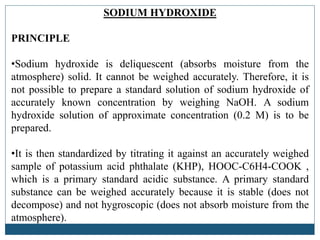
To standardize a solution of NaOH, a substance with a known acidity, such as potassium hydrogen phthalate (KHP), is used as a primary standard. A primary standard is a highly pure and stable substance that can be accurately weighed and dissolved to prepare a solution of known concentration.
The process of standardizing a solution of NaOH involves the following steps:
Weigh out a known amount of KHP and transfer it to a clean, dry flask.
Add a small amount of distilled water to the flask and dissolve the KHP to create a standard solution.
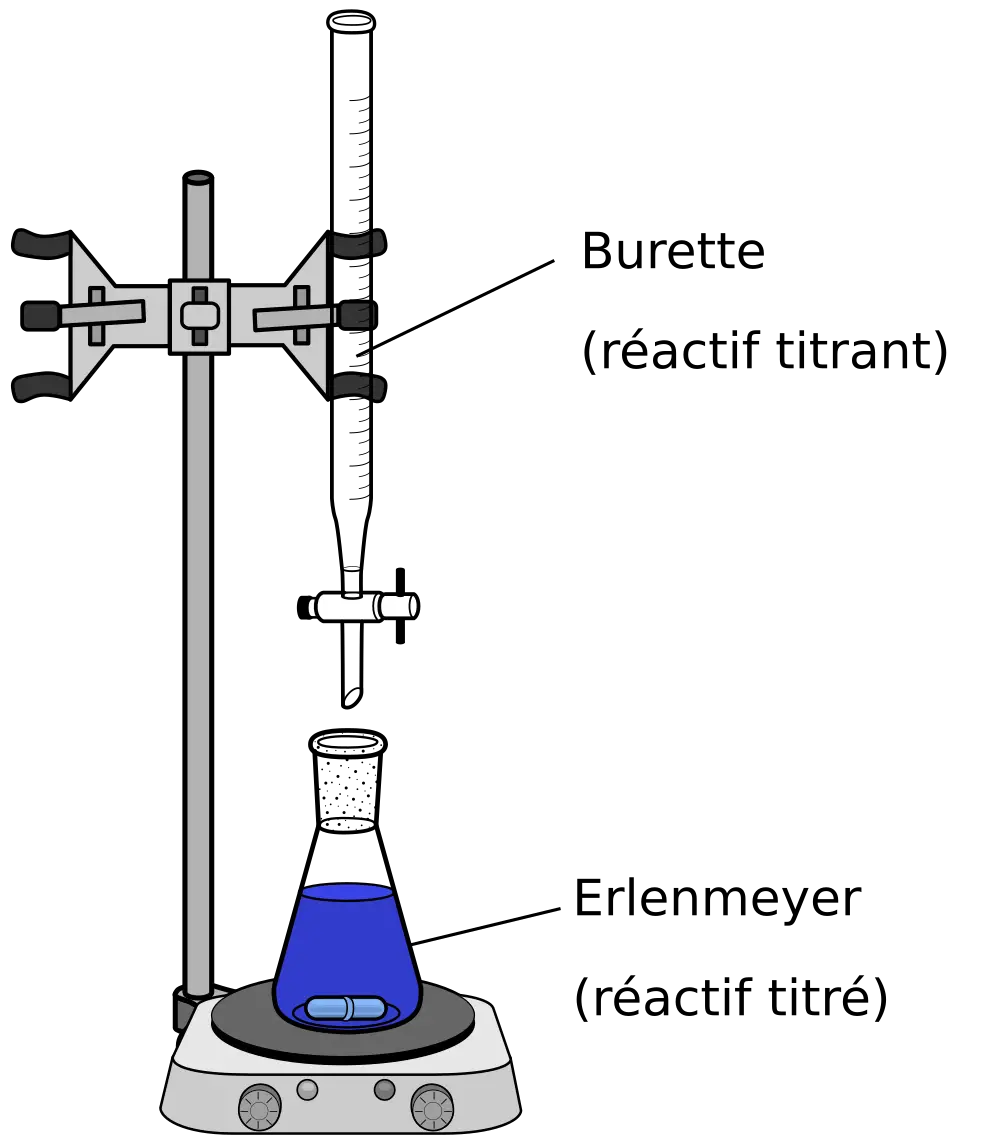
Using a burette, a piece of laboratory equipment used to measure and dispense precise volumes of liquids, accurately measure and dispense a known volume of the NaOH solution into a conical flask.
Add a few drops of an indicator, such as phenolphthalein, to the conical flask. This will change color when the solution becomes neutral or slightly alkaline, indicating that the acid has been neutralized by the base.
Slowly add the standard KHP solution to the conical flask, using a pipette or burette, until the indicator changes color. This is known as the endpoint of the titration.
Record the volume of the KHP solution used at the endpoint.
Calculate the concentration of the NaOH solution using the following formula:
Concentration (M) = (Weight of KHP / Molecular weight of KHP) / Volume of KHP used (L)
Standardizing a solution of NaOH is important because it allows users to accurately measure the concentration of the solution, which is necessary for precise and reliable results in various applications. In addition, standardizing a solution ensures that it is of consistent quality and strength, which is essential for the reproducibility of experimental results.
In conclusion, standardization of NaOH is the process of determining the concentration of a solution of the strong base using a primary standard substance. It is an important step in ensuring the accuracy and reliability of results in various industrial and laboratory settings.
Preparation and Standardization of 0.1 M Sodium Hydroxide

The indicator, phenolphthalein, turns the solution from clear to pink in a basic solution. Which means that when HCl reacts with NaOH,it needs one mole to neutralize the NaOH, while the H2SO4 needs only ½ a mole to neutralize the same. After finding the mean of the concentration, the standard deviation was found to be 0. To visually find the end point. NaOH was standardized using a primary standard KHP, by titration, this is an excellent method to prepare a secondary standard because of its high level of accuracy. At the end of this experiment, student found that a diluted sodium hydroxide takes more time to standardize when titrating the solution in present of light pale pink compare to pure sample of sodium hydroxide solution. Now, we have got a complete detailed explanation and answer for everyone, who is interested! This indicator shows colour change according to the concentration of hydrogen ions or pH.
Preparation and standardization of sodium hydroxide

Therefore, it is not possible to prepare a standard solution of sodium hydroxide of accurately known concentration by weighing NaOH. However, as NaOH was added further, there came a point when no amount of stirring changed the pink colour. KHP was used because it is of high purity, high molar mass which yields lower massing errors and reacts well with the solution it is titrated with. Two theories are given below to explain the colour change. To determine the concentration, standardization has to be used to find the concentration of the solution. For every one mole of KHP, it would take one mole of NaOH to react it completely.
Standardization of NaOH and blog.sigma-systems.com

When the solid KHP is dissolved in water and a base of sodium hydroxide is added by titration 2 , the reaction will get to a point where its neutralized, that means that the reaction has met its end point. Standardization of sodium hydroxide NaOH. Question 2: Why might mass measurements using an analytical balance to measure about 25 g of water be considered more accurate than a volume measurements of 25 mL with volumetric glassware, such as burets or transfer pipets? This report is about how to standardize a Sodium Hydroxide NaOH solution by titrating it with pure sample of Potassium acid Phthalate KHC8H4O4. Standardization of Sodium Hydroxide NaOH solution. KHP50 milliliter Graduated cylinderDistilled WaterTitration Set Up SpatulaBurette Top lading balance ± 0. Volume of Oxalic acid taken ml Burette reading of sodium hydroxide ml Volume of Sodium hydroxide consumed ml IR FR 1. Once the concentration of NaOH is found, it will help to find the percent purity of an unknown KHP.