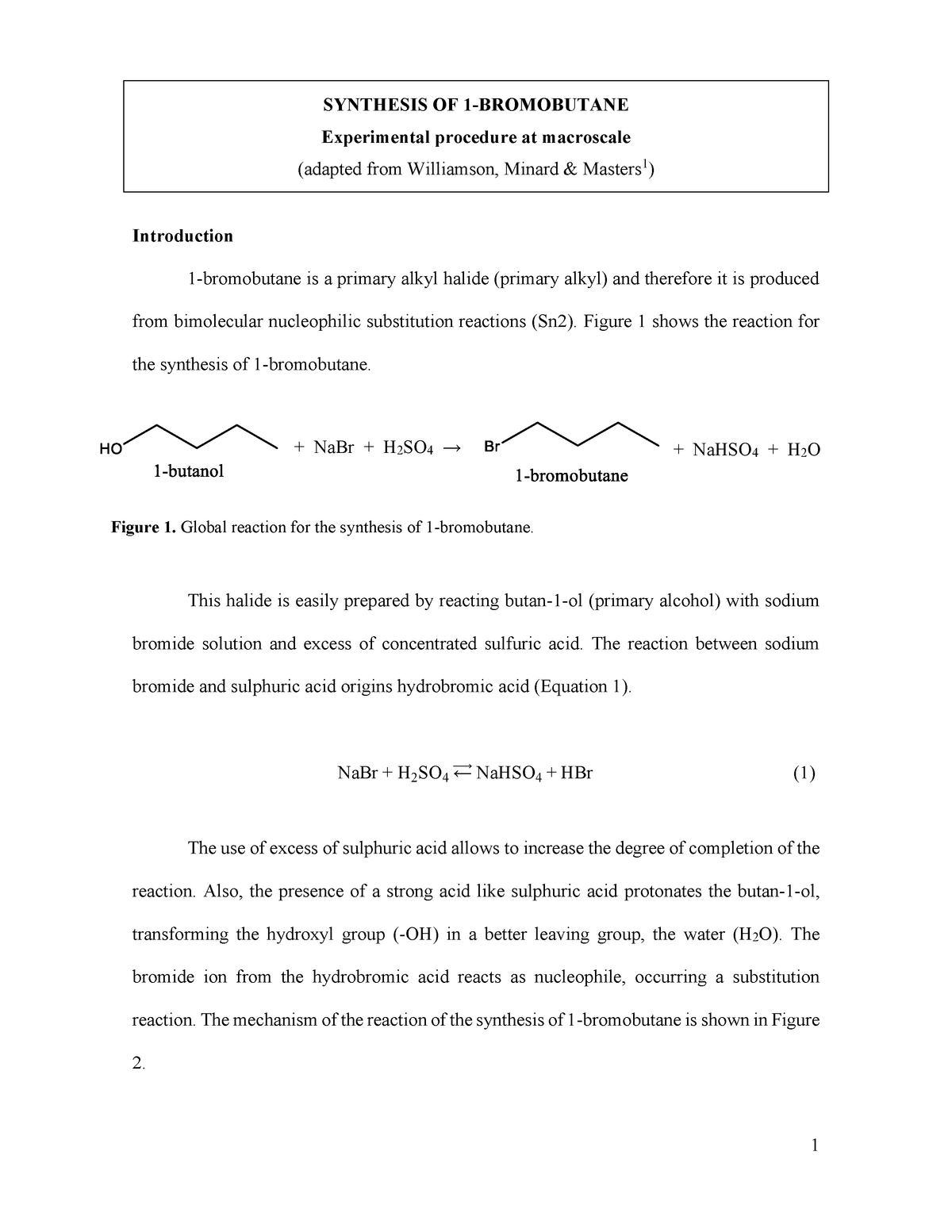
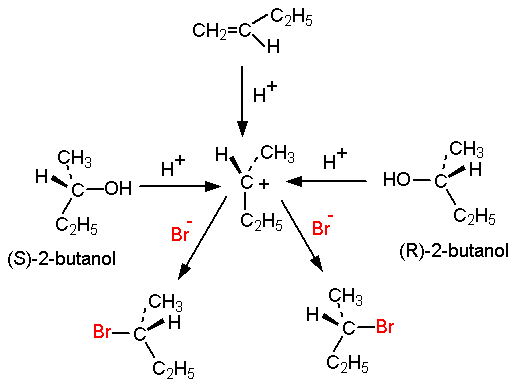
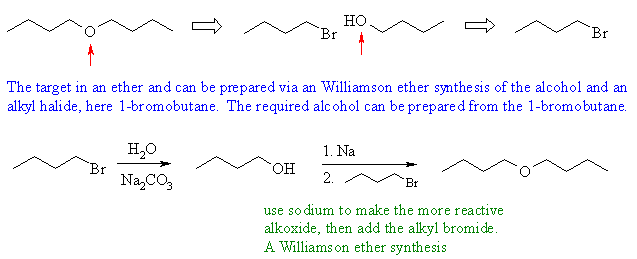
Synthesis of 1-bromobutane from 1-butanol is a common experiment in undergraduate chemistry labs. It involves the conversion of a primary alcohol, 1-butanol, into a primary alkyl halide, 1-bromobutane, through an SN2 substitution reaction.
The reaction is typically carried out in an aqueous solution of hydrobromic acid (HBr) and requires the presence of a nucleophile, such as water, to facilitate the substitution. The alcohol is first protonated by the acid, forming a stable intermediate called an alkyloxonium ion. The nucleophile then attacks the electrophilic carbon atom of the alkyloxonium ion, displacing the bromide ion and forming the desired alkyl halide.
One of the key considerations in the synthesis of 1-bromobutane is the choice of solvent. Water is a commonly used solvent, but other polar solvents such as ethanol or methanol can also be used. The choice of solvent can affect the rate of the reaction, as well as the yield and purity of the final product.
The reaction can be conducted in either the gas phase or the liquid phase, depending on the desired outcome. In the gas phase, the reaction proceeds faster but the yield is typically lower due to the greater likelihood of side reactions. In the liquid phase, the reaction proceeds slower but the yield is typically higher due to the lower likelihood of side reactions.
The yield of 1-bromobutane can also be affected by the ratio of reactants used. Generally, a large excess of HBr is used to ensure complete conversion of the alcohol to the alkyl halide. However, using too much HBr can lead to the formation of unwanted side products, such as alkyl bromides and alkyl halides.
The purity of the final product can be determined by various methods, including gas chromatography and infrared spectroscopy. These techniques allow for the identification and quantification of impurities present in the final product, which can be used to optimize the reaction conditions and improve the overall yield.
In summary, the synthesis of 1-bromobutane from 1-butanol is a widely studied reaction in undergraduate chemistry labs. It involves the conversion of a primary alcohol into a primary alkyl halide through an SN2 substitution reaction, and the choice of solvent, reaction phase, and reactant ratio can all affect the yield and purity of the final product.