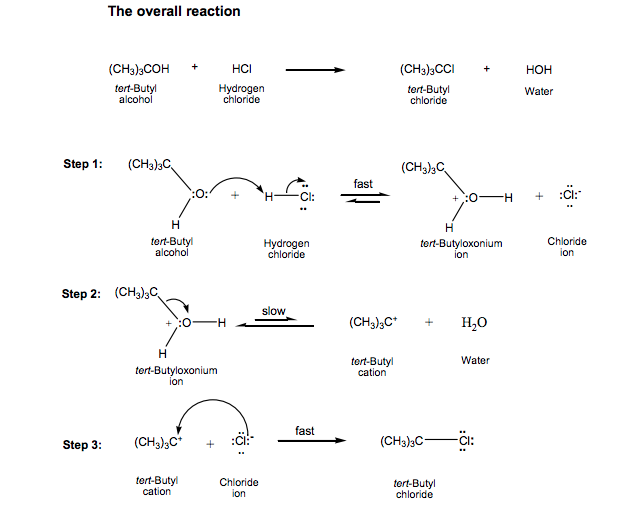
Tert-butyl chloride, also known as t-butyl chloride or t-BuCl, is a chemical compound with the formula C4H9Cl. It is a colorless liquid with a pungent, sweet odor, and is commonly used as a solvent and as a starting material in the production of other chemicals.
One important property of tert-butyl chloride is its solubility. Solubility refers to the ability of a substance to dissolve in a solvent to form a homogenous mixture. The solubility of a substance is influenced by several factors, including the nature of the solvent and the temperature.
In general, tert-butyl chloride is highly soluble in many common solvents. It is soluble in water, but only to a limited extent, with a solubility of about 0.14 g/100 mL at 20°C. This means that only a small amount of tert-butyl chloride will dissolve in water at room temperature.
Tert-butyl chloride is more soluble in polar solvents such as alcohols and ethers. It is highly soluble in methanol, ethanol, and isopropyl alcohol, with solubilities ranging from 40 to 60 g/100 mL at 20°C. It is also highly soluble in diethyl ether, with a solubility of about 120 g/100 mL at 20°C.
In addition to its solubility in various solvents, the solubility of tert-butyl chloride can also be influenced by temperature. Like many other chemicals, tert-butyl chloride becomes more soluble as the temperature increases. This is due to the fact that increasing the temperature can increase the kinetic energy of the solvent molecules, allowing them to more effectively dissolve the solute.
In summary, tert-butyl chloride is a highly soluble chemical compound that readily dissolves in polar solvents such as alcohols and ethers. Its solubility can also be influenced by temperature, with an increase in temperature leading to an increase in solubility.