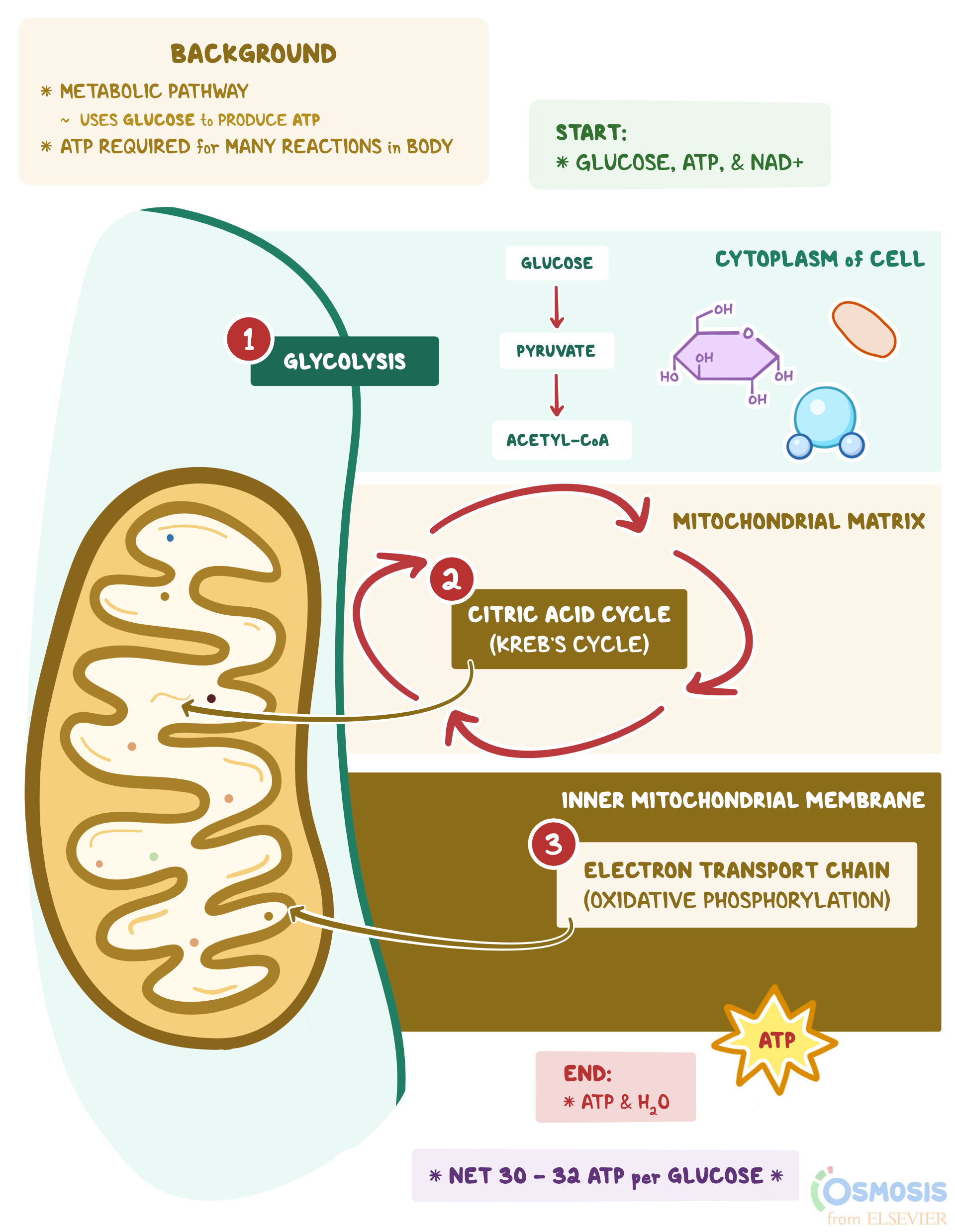
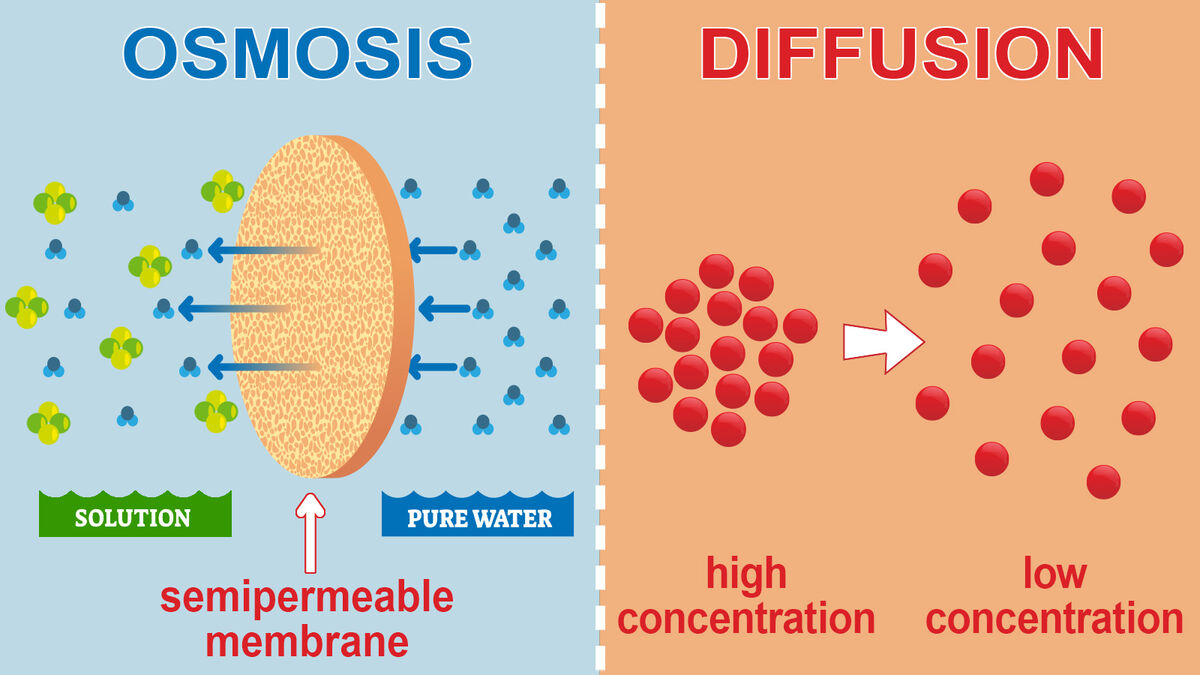
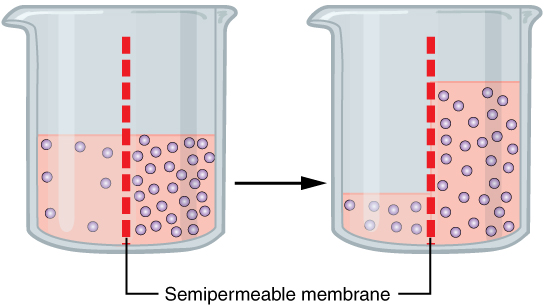
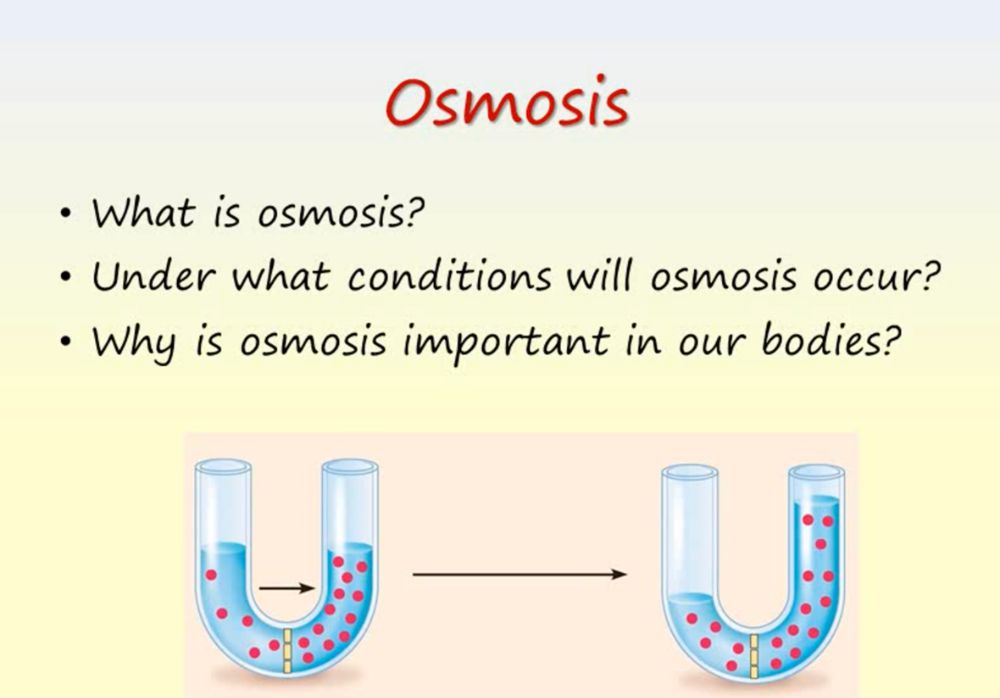
Osmosis is the diffusion of water through a semi-permeable membrane, and it is an essential process for the proper functioning of cells in living organisms. Osmosis plays a crucial role in maintaining the balance of water and solutes inside and outside of cells, which is necessary for the survival and proper functioning of cells.
One of the primary functions of osmosis is to regulate the concentration of solutes in cells. All cells have a semi-permeable membrane that allows certain substances to pass through while preventing others from entering. This membrane helps to maintain the proper balance of water and solutes inside the cell, which is essential for the cell's metabolic processes.
For example, cells need a specific concentration of ions and other solutes to function properly. If the concentration of solutes becomes too high or too low, the cell may become damaged or even die. Osmosis helps to maintain the proper balance of solutes by allowing water to move in or out of the cell as needed.
In addition to regulating the concentration of solutes, osmosis also helps to maintain the proper volume of cells. Cells need a specific volume in order to function properly, and osmosis plays a crucial role in maintaining this volume. If the concentration of solutes outside the cell is higher than inside, water will move out of the cell through osmosis, causing the cell to shrink. Conversely, if the concentration of solutes outside the cell is lower than inside, water will move into the cell through osmosis, causing the cell to swell.
Osmosis is also important for the transport of nutrients and waste products in and out of cells. Nutrients and waste products are dissolved in water, and osmosis helps to move these substances across the cell membrane. For example, when a cell absorbs nutrients from its surroundings, osmosis helps to transport these nutrients across the cell membrane into the cell. Similarly, when a cell needs to eliminate waste products, osmosis helps to transport these waste products out of the cell and into the surrounding environment.
In summary, osmosis is an essential process for the proper functioning of cells in living organisms. It helps to regulate the concentration and volume of cells, as well as transport nutrients and waste products in and out of cells. Without osmosis, cells would be unable to maintain the proper balance of water and solutes, and they would be unable to function properly.