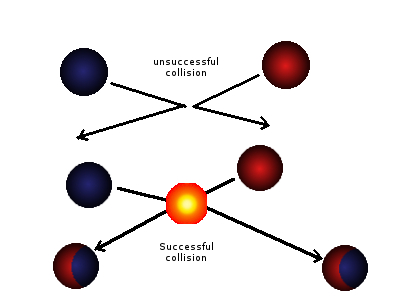
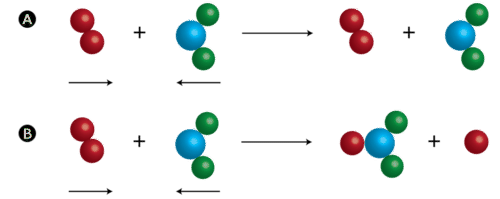
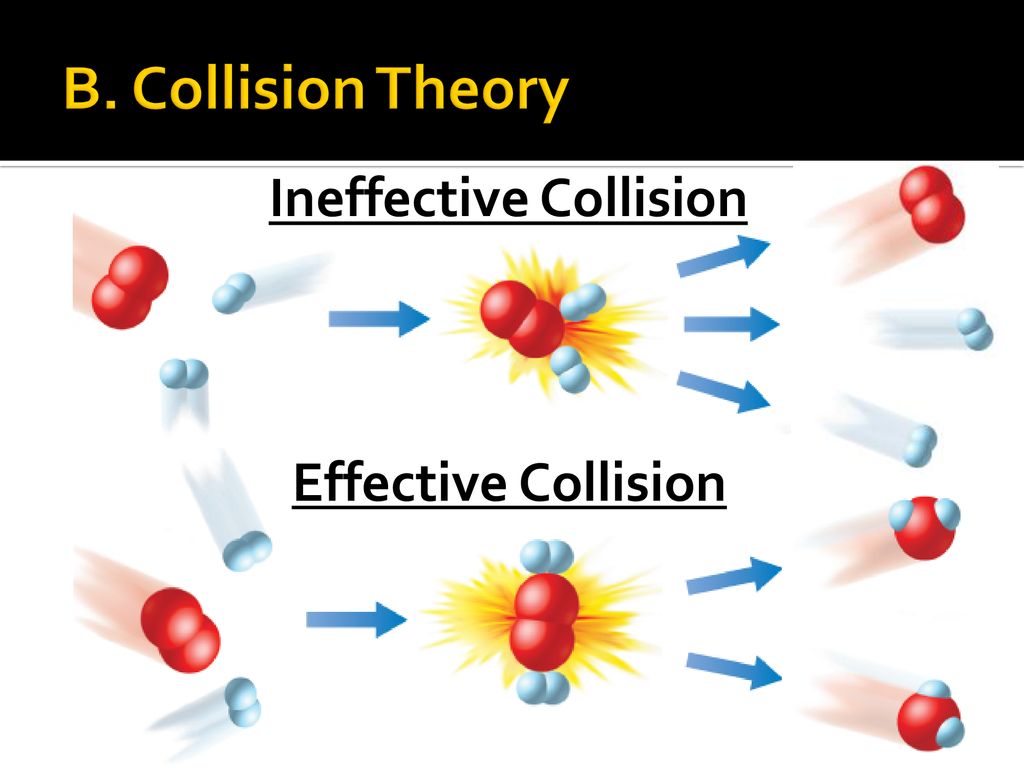
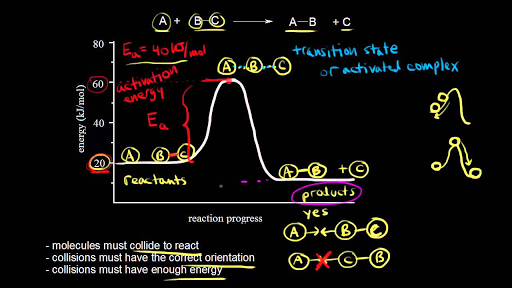
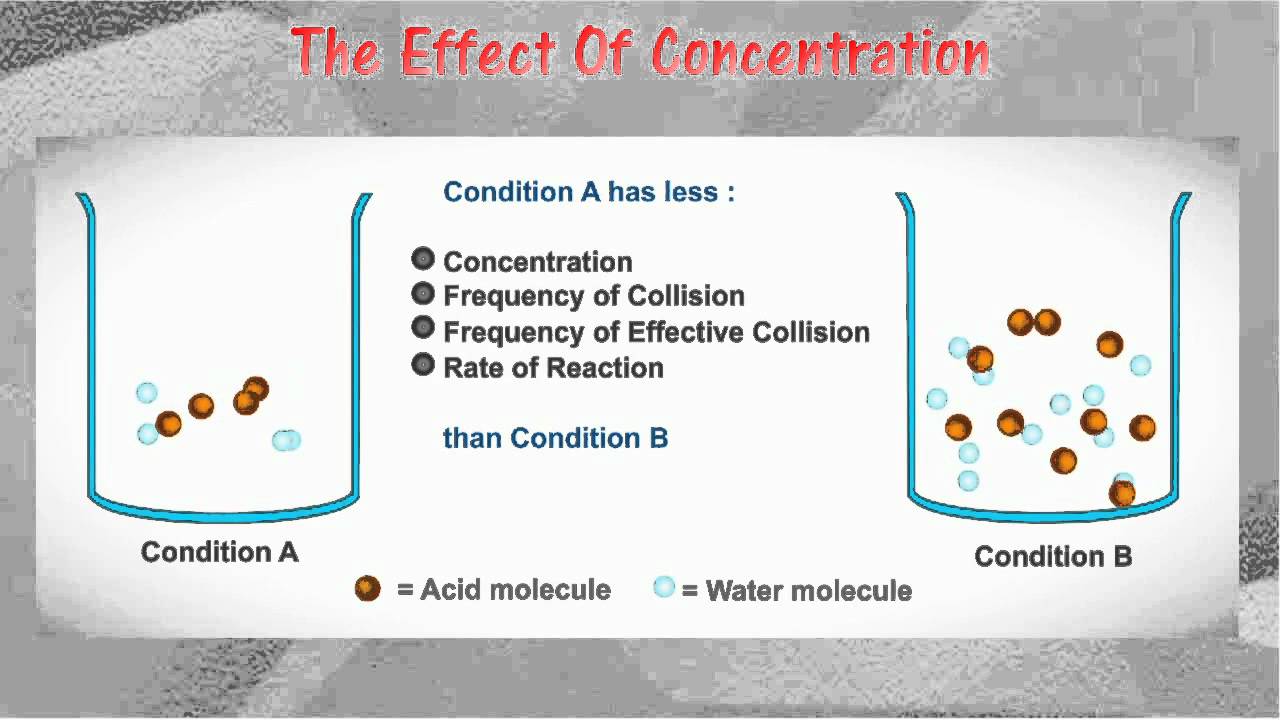
Collision theory is a scientific theory that explains the rates of chemical reactions by examining the frequency, energy, and nature of collisions between reactant molecules. According to this theory, a chemical reaction will occur only if the reactant molecules collide with enough energy and in the correct orientation to overcome the activation energy barrier and form the products of the reaction. In other words, the likelihood of a chemical reaction occurring is dependent on the frequency and energy of collisions between reactant molecules, as well as their orientation relative to each other.
One classic example of collision theory in action is the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O). In order for this reaction to occur, the hydrogen and oxygen molecules must collide with sufficient energy and in the correct orientation to overcome the activation energy barrier and form a water molecule. If the reactant molecules do not have enough energy or the correct orientation, they will simply bounce off each other and no reaction will occur.
To understand how collision theory applies to this reaction, let's consider the energy required for the reaction to occur. In order for the hydrogen and oxygen molecules to form a water molecule, they must have enough energy to break the bonds between the atoms in the reactant molecules and form new bonds between the atoms in the product molecules. This energy is known as the activation energy of the reaction, and it is represented by the height of the activation energy barrier in the energy diagram for the reaction.
If the reactant molecules do not have enough energy to overcome the activation energy barrier, they will simply bounce off each other and no reaction will occur. However, if the reactant molecules have enough energy to overcome the activation energy barrier, they will collide and form the products of the reaction. In the case of the hydrogen-oxygen reaction, this means that the hydrogen and oxygen molecules will combine to form a water molecule.
In addition to energy, the orientation of the reactant molecules relative to each other also plays a role in the likelihood of a chemical reaction occurring. In order for the reaction between hydrogen and oxygen to occur, the hydrogen and oxygen molecules must collide in the correct orientation so that the atoms can bond together and form the water molecule. If the molecules collide in the wrong orientation, they will simply bounce off each other and no reaction will occur.
Overall, collision theory helps us understand the rates of chemical reactions by examining the frequency, energy, and nature of collisions between reactant molecules. By understanding these factors, we can predict the likelihood of a chemical reaction occurring and design experiments to study the reaction process in more detail.