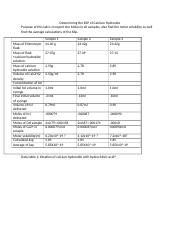
Solubility product, or Ksp, is a measure of the concentration of ions in a saturated solution of a sparingly soluble salt. It is an important parameter in determining the behavior of a salt in solution, including its potential to precipitate out of solution under certain conditions. The solubility product of calcium hydroxide, Ca(OH)2, can be determined through titration, a common laboratory technique used to determine the concentration of a substance in solution.
To determine the solubility product of calcium hydroxide through titration, a known concentration of hydrochloric acid (HCl) is added to a solution of calcium hydroxide until the point of neutralization is reached, at which point all of the hydrogen ions (H+) from the hydrochloric acid have reacted with the hydroxide ions (OH-) from the calcium hydroxide. The concentration of HCl is known, and the volume of acid added can be measured accurately using a burette. From the concentration and volume of HCl used, the number of moles of H+ ions that have reacted with the OH- ions can be calculated.
The solubility product of calcium hydroxide can then be determined using the following equation:
Ksp = [Ca2+][OH-]2
Where [Ca2+] and [OH-] represent the concentration of calcium ions and hydroxide ions in the solution, respectively. The concentration of these ions can be calculated using the number of moles of H+ ions that have reacted with the OH- ions and the volume of the solution.
It is important to note that the solubility product of a salt is temperature dependent, so the temperature of the solution should be carefully controlled during the titration process to ensure accurate results. Additionally, it is essential to use a high-purity form of calcium hydroxide and hydrochloric acid to minimize the impact of impurities on the solubility product calculation.
In conclusion, the solubility product of calcium hydroxide can be accurately determined through titration, a widely used laboratory technique that involves the addition of a known concentration of acid to a solution of the salt. By carefully controlling the temperature of the solution and using high-purity reagents, the solubility product can be determined with good accuracy. This information is useful in understanding the behavior of calcium hydroxide in solution and predicting its potential to precipitate out under different conditions.
ChemTeam: Calculate Ksp when Given Titration Data

Freeman: New York, 2006, p. It does not matter if it overfills to just above the 0cm mark because the excess can be drained off into a beaker, by opening the stopcock. Place the conical flask containing the calcium hydroxide underneath the burette. This implied that the dissolving and crystallization reactions happened at an equal rate and simultaneously. Now we know the amount of moles in 25cm we can calculate the amount of moles of calcium hydroxide there would be in 1dm. Ksp 4 x10-5 4 x 10- Average Ksp of Ca OH 2 4 x 10-5 4 x 10- Table value of Ksp Ca OH 2 5 x 10-6 5 x 10- Conclusion: The purpose of this experiment, which was to determine the solubility product constant, Ksp, of a calcium hydroxide by titration of dissolved hydroxide, was achieved.
Determination of the Solubility Product Constant of...

It was observed that as the ionic strength increases, the solubility increases. At this point it is essential to calculate the molarity of the acid that will be used in the titration. Quezon City: University of the Philippines Diliman, 2011. In this experiment, part A of the lab will be run with Ca OH 2 at room temperature and in part A it will be heated up. Read the last paragraph, I mentioned every concept in detail including everything you mentioned in your last two posts; three alternatives to express solubility. Once the color change occurs, boil the sample for 2 to 3 minutes, then cool to room temperature.
Determination of the solubility of calcium hydroxide

The first of these words is isotonic. Furthermore, by using Equation 12 as shown in the Appendix, the theoretical value for the solubility was also taken, which is about 0. Close the stopcock and fill the burette with the hydrochloric acid, using the funnel. Physical Chemistry, 8 th Ed. Take a 25cm sample of the calcium hydroxide solution by using a 25cm pipette and a triple valve rubber bulb pipette filler. Basically, Ksp is the quantification of the relationship of the ionic solid and its constituent ions.
Exp 4 Determining the Solubility Product Constant (ksp) of Calcium Hydroxide

It is necessary now to calculate the formula mass of Calcium hydroxide. McCormick Last Update: Ocober 13, 2013 Introduction An equilibrium constant, K, is related to Δ G for a process through Eqn. The solubility would be 0. The first two were decanted, while the third was poured through a filter together with the precipitate. The experiment shows the converse of the diverse-ion effect. After the tip is immersed into the liquid , the operating button is released. Quickly gravity filter each solution to remove excess Ca OH 2 such that the temperature of the solution does not change appreciably during the filtration.
Determination of solubility product of calcium hydroxide by titrating with standard hydrochloric acid

Calculate the concentration of OH¯, Pb 2+ and the K sp of this satured solution. Once the titration began, the HCl was added until the methyl orange endpoint was reached, and as a result the volume of the HCl needed for the endpoint to be reached could be used in determining the moles and in turn the molar solubility and the solubility constant of the Ca OH 2. When the data of the KCl media were plotted, it showed that the solutions decreased in solubility through increasing ionic strength. Purpose The purpose of this experiment is to determine the Ksp value of aqueous calcium hydroxide by mixing it with a solution of hydrochloric acid with a known molarity until equilibrium is reached. The colour of the methyl orange now is a yellowy colour. A saturated, aqueous, solution of Ca OH 2 is represented in equation form as shown below. Carbonate-free water is prepared by boiling high-purity water either distilled or prepared by reverse osmosis followed by ion exchange and filtration under inert gas purge and then cooling under inert gas.