A final draft is the last version of a piece of writing that has been revised and edited. It is the version that will be submitted, published, or otherwise shared with others.
The key to a successful final draft is thorough editing and revision. This process involves not only correcting grammar and spelling errors, but also ensuring that the writing is clear, concise, and well-organized. It may involve rearranging or deleting sections, adding transitional phrases or sentences, and clarifying ideas.
One important aspect of the final draft process is getting feedback from others. This can be from a writing partner, a peer, a teacher, or a professional editor. Other people can often spot problems or suggest improvements that the writer might have missed. It is important to be open to constructive criticism and willing to make changes based on the feedback received.
Another key to a successful final draft is setting aside enough time to properly revise and edit the writing. This can be a time-consuming process, but it is worth the effort to produce a polished and professional final product.
Overall, the key to a successful final draft is careful and thorough editing and revision, seeking feedback from others, and setting aside enough time to do the job well. By following these steps, writers can produce a final draft that is clear, concise, and well-organized, and that effectively communicates their ideas to their intended audience.
Diels
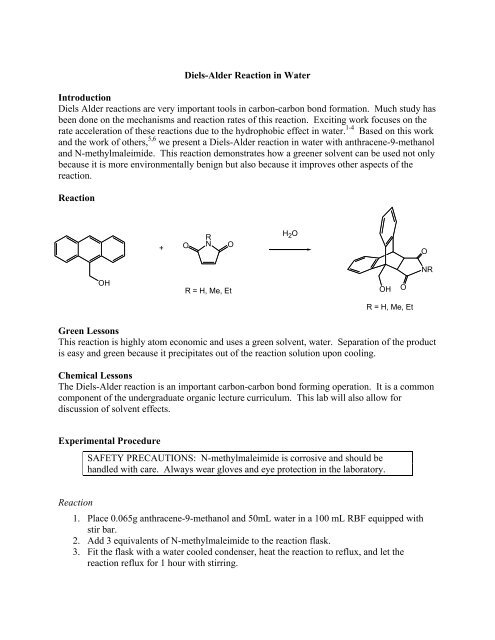
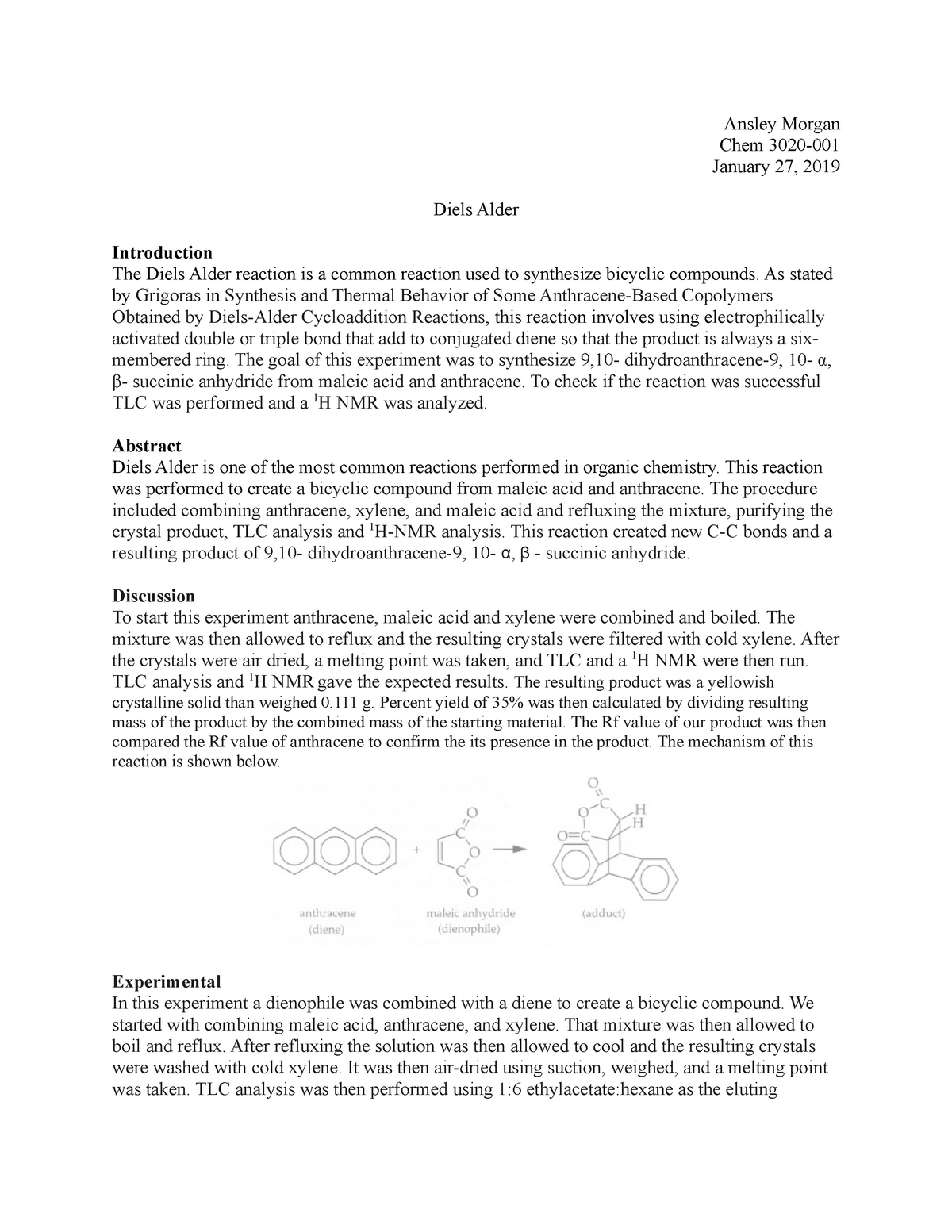
Residual water appeared to have contributed to the % yield being over 100%. A Sn2 reaction was conducted; this involved benzyl bromide, sodium hydroxide, an unknown compound and ethanol through reflux technique, mel-temp recordings, recrystallization, and analysis of TLC plates. The triphenylmethanol looked like a white powder. About 12 mL of deionized water was added to about 2 grams of anhydride in an Erlenmeyer flask. For the following experiment, the main focus is the use of a cycloaddition reaction, also known as Diels-Alder. The melting point analysis shows that the product of the Diels-Alder reaction was somewhat impure, but the product of the hydrolysis of the Diels-Alder adduct was very pure. As expected, as the product was further reacted as in the hydrolysis of the anhydride it became more purified.
Lab blog.sigma-systems.com

The results would be shown by the support of two graphs. After crystallization begins, cool the flask in an ice-water bath to complete the process, collect the solid by vacuum filtration, and air-dry it. During drying, the anhydride was exposed to the air. When the Diels-Alder reaction was complete, there was. The melting point test showed that the product was impure, however.
Diels Alder Reaction Lab report OChem

The evidence from the qualitative testing performed in the laboratory confirms the formation of six-membered rings by cycloaddition, also known as a Diels-Alder reaction. The purpose of this lab is to demonstrate the formation of six-membered ring s by a cyclo addition reaction. To ensure good yield, both butadiene and maleic anhydride must be in the same physical phase, which means that either the experiment takes place at extremely low temperature below the melting point of butadiene, -108. The purity of the anhydride was slightly in question because of the 3 degree difference and its pale-yellow, white color. Reactions: Procedure: The reaction mixture was created.