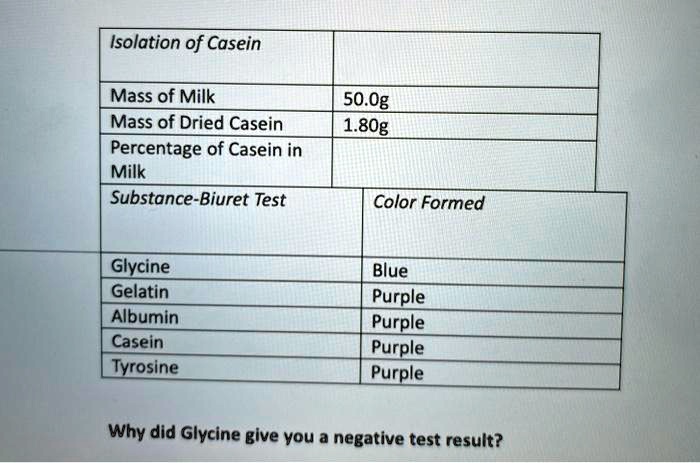
Milk is a complex liquid that contains a variety of proteins, carbohydrates, lipids, and minerals. Two of the most important components of milk are casein and lactose. Casein is a type of protein that makes up about 80% of the protein in milk and is responsible for giving milk its characteristic thickness and clabbiness. Lactose, on the other hand, is a type of carbohydrate that makes up about 4-5% of the total solids in milk and is the primary source of energy for newborn mammals.
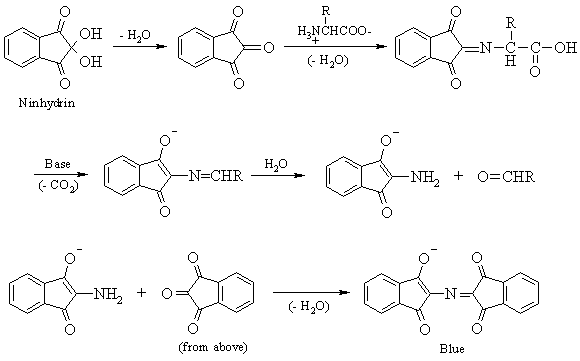
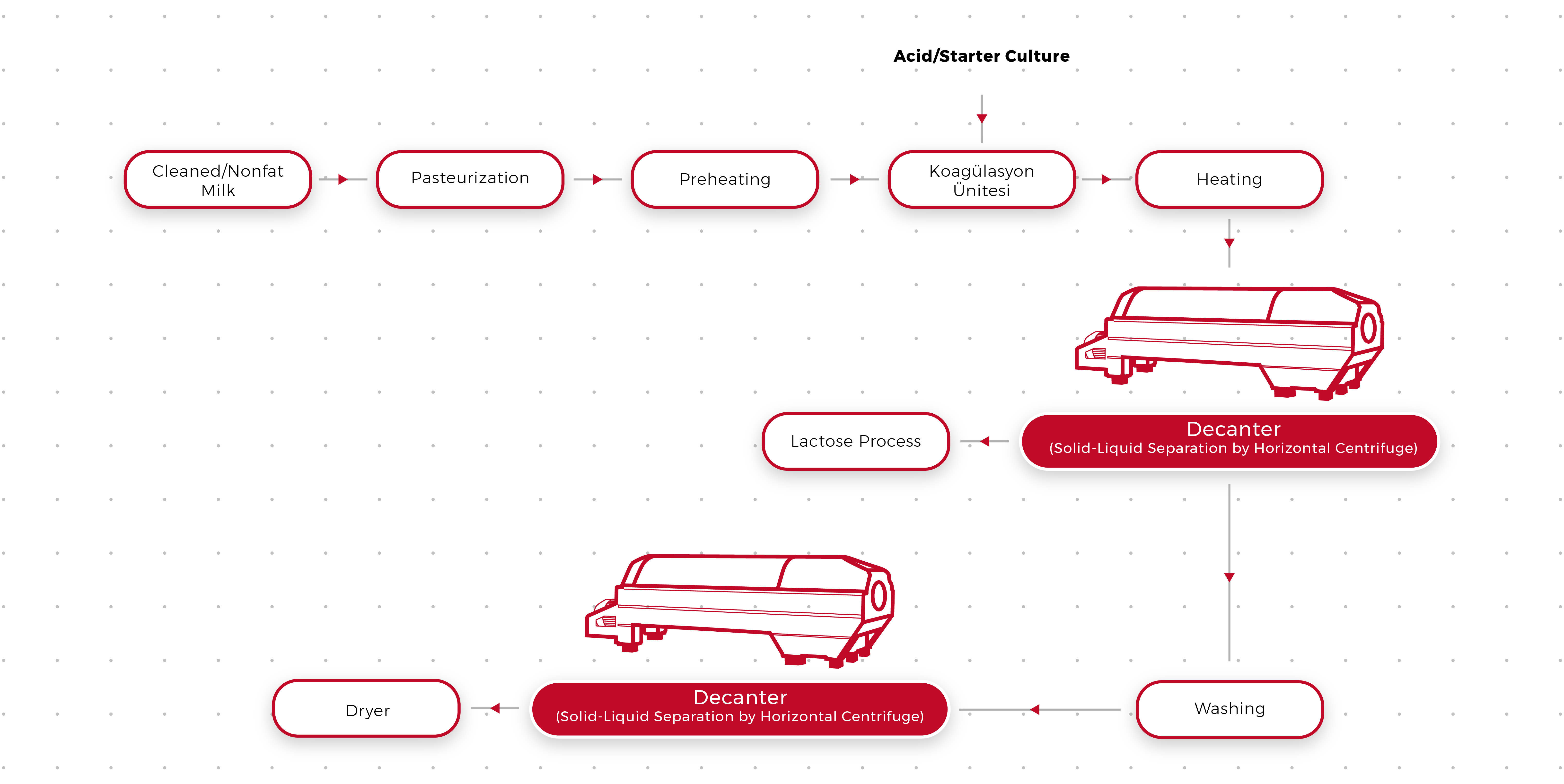
There are several methods that can be used to isolate casein and lactose from milk. One of the most common methods is called acid precipitation, which involves the use of an acid to cause the proteins in milk to coagulate and form a solid curd. To isolate casein using this method, milk is first acidified by the addition of an acid like hydrochloric acid or sulfuric acid. As the acidity of the milk increases, the proteins in the milk begin to coagulate and form a solid curd. The curd is then separated from the liquid whey by filtering or centrifugation, and the casein is recovered from the curd.
Another method for isolating casein from milk is called alkaline precipitation, which involves the use of an alkaline solution to cause the proteins in milk to coagulate. To isolate casein using this method, milk is first treated with an alkaline solution like sodium hydroxide or potassium hydroxide. As the pH of the milk increases, the proteins in the milk begin to coagulate and form a solid curd. The curd is then separated from the liquid whey and the casein is recovered from the curd in the same way as in the acid precipitation method.
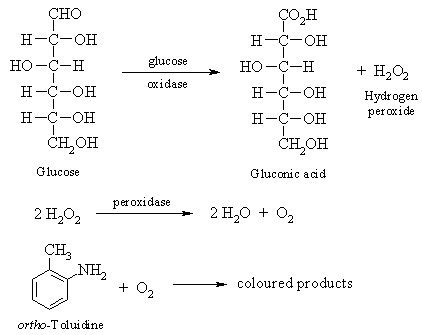
Lactose can also be isolated from milk using a variety of methods. One common method is called lactase hydrolysis, which involves the use of the enzyme lactase to break down the lactose in milk into its component sugars, glucose and galactose. To isolate lactose using this method, milk is first treated with lactase to hydrolyze the lactose into glucose and galactose. The glucose and galactose are then separated from the other components of the milk by filtration or centrifugation, and the lactose is recovered from the filtrate or supernatant.
Other methods for isolating lactose from milk include precipitation with alcohol, ion exchange chromatography, and membrane filtration. Regardless of the method used, the isolation of casein and lactose from milk is an important step in the production of a wide range of dairy products, including cheese, yogurt, and ice cream.
In conclusion, casein and lactose are two important components of milk that can be isolated using a variety of methods, including acid precipitation, alkaline precipitation, lactase hydrolysis, and others. The isolation of these components is important for the production of a wide range of dairy products and has a significant impact on the food industry.