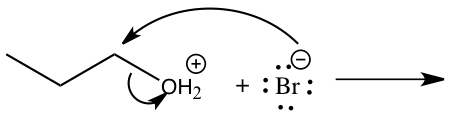
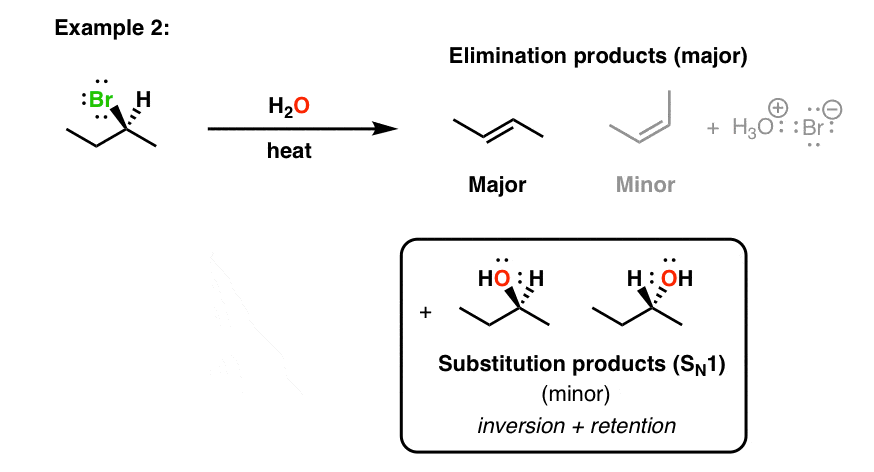
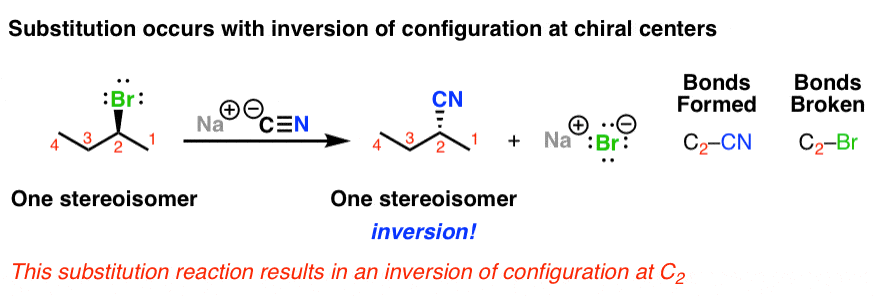
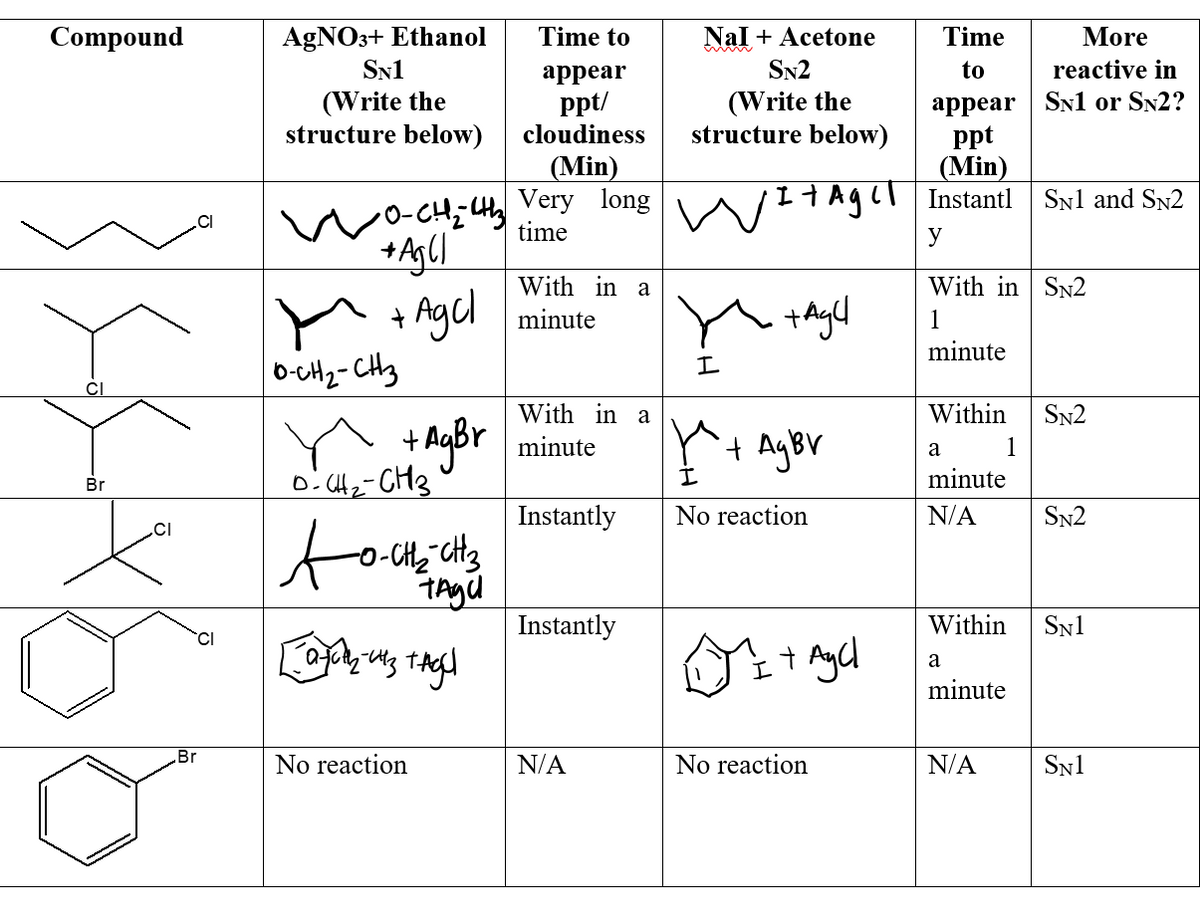
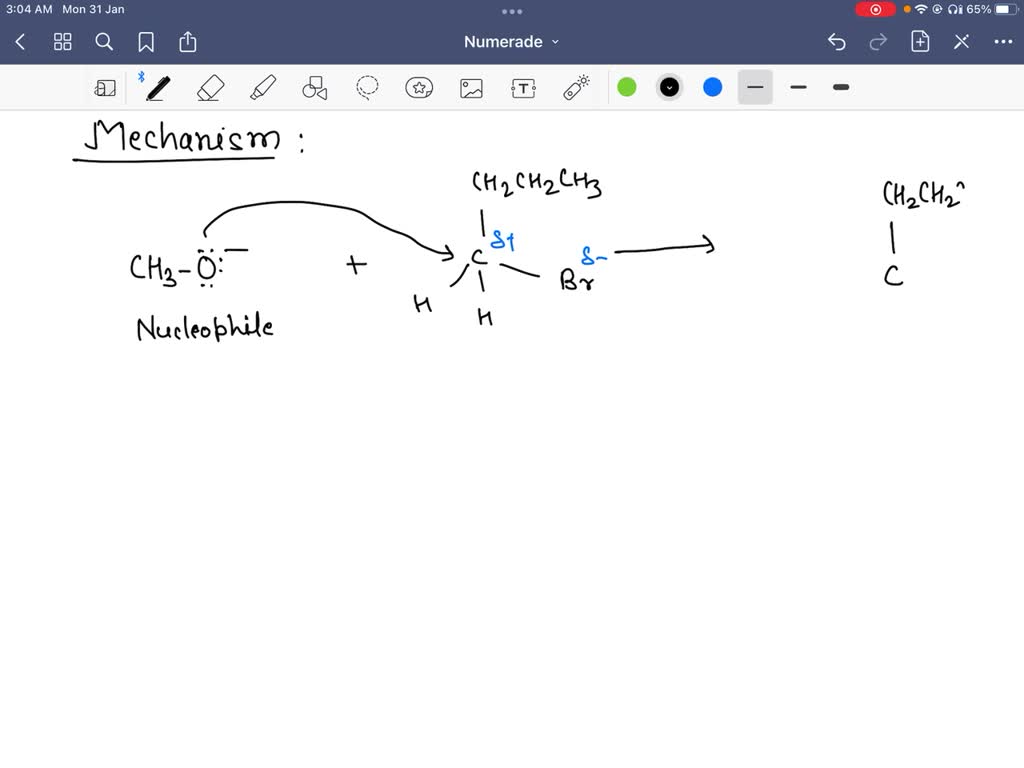
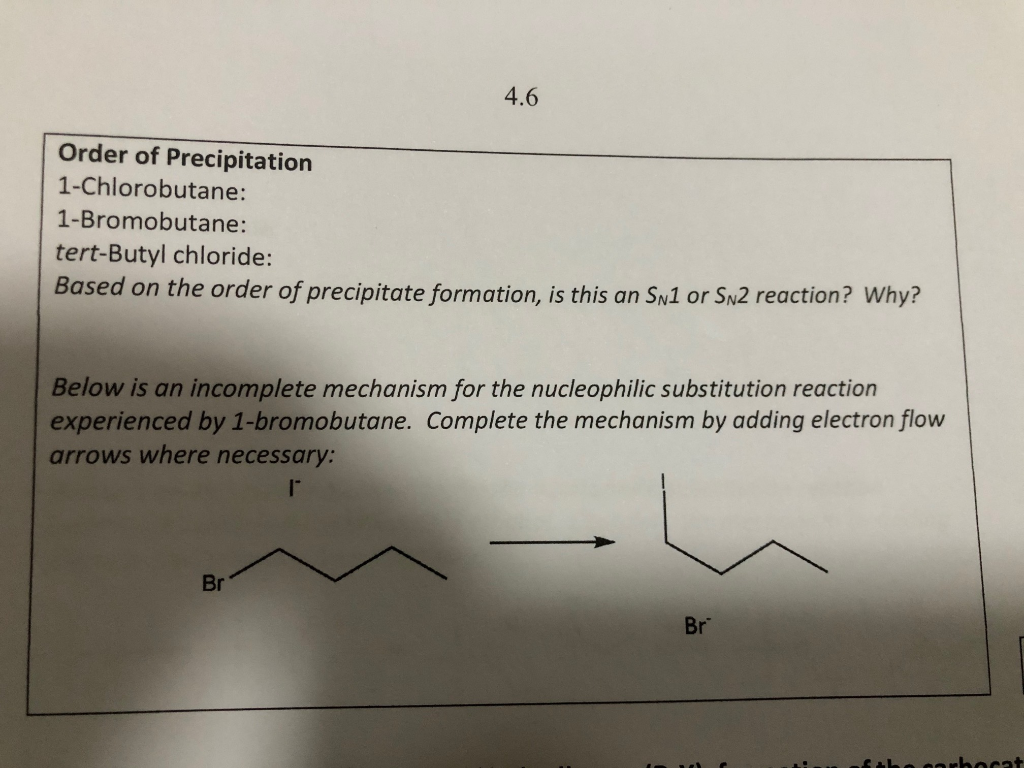
Queen Sheba, also known as Bilqis, was a powerful and influential queen who ruled over the kingdom of Sheba, located in what is now modern-day Yemen and Ethiopia. She is described in various ancient texts as a wealthy and influential ruler, known for her wisdom and wealth.
According to the biblical account, Queen Sheba visited King Solomon in Jerusalem and was so impressed by his wisdom and wealth that she brought him gifts of gold, spices, and precious stones. In the Islamic tradition, Queen Sheba is also revered as a wise and just ruler who was beloved by her people.
Throughout history, Queen Sheba has been depicted as a symbol of female power and leadership, and her story has inspired countless works of art, literature, and music. In recent years, she has gained renewed attention as a figure of empowerment and inspiration for women.
Despite the fact that she lived many centuries ago, Queen Sheba's legacy lives on today as a symbol of female strength and leadership. She serves as an inspiration to women everywhere, reminding us that we are capable of achieving great things and making a positive impact on the world.
In conclusion, Queen Sheba was a powerful and influential queen who left a lasting legacy as a symbol of female strength and leadership. Her story continues to inspire and empower women today, reminding us that we are capable of achieving great things and making a positive impact on the world.