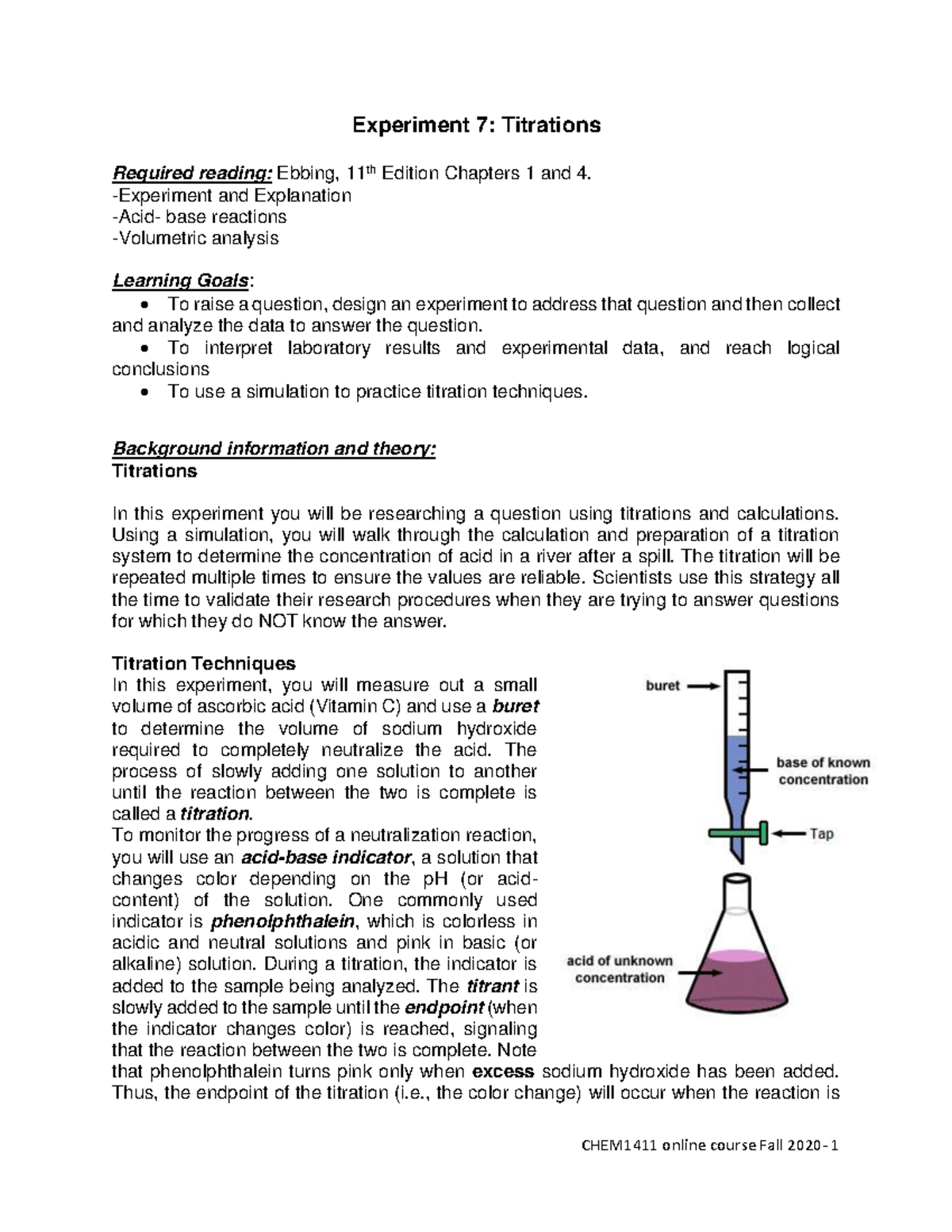
A titration is a laboratory technique that is used to determine the concentration of a substance, typically an acid or a base, in a solution. It involves the use of a buret, which is a long, thin tube with a valve at one end and a measuring scale at the other. A known volume of the substance of interest is placed in the buret, and a solution of known concentration, called the titrant, is added to the substance. The titrant is typically a base if the substance of interest is an acid, or an acid if the substance of interest is a base.
The process of titration involves adding the titrant to the substance of interest in small increments, called aliquots, until the point of chemical equivalence is reached. This point is known as the endpoint of the titration, and it is marked by a change in the color of an indicator solution that is added to the substance of interest. The endpoint indicates that the acid and base have neutralized each other, forming a neutral solution.
In a titration lab report, the discussion section is used to interpret and analyze the results of the experiment. It is important to ensure that the results are accurate and reliable, and to identify any sources of error that may have occurred during the experiment.
One common source of error in titration experiments is the use of impure reagents. It is important to ensure that the reagents used in the experiment are pure, as impurities can affect the accuracy of the results. Another potential source of error is the use of incorrect equipment, such as a buret that is not properly calibrated. It is also important to ensure that the endpoint of the titration is accurately determined, as this can affect the accuracy of the results.
In the discussion section of a titration lab report, it is also important to compare the results of the experiment to theoretical values. This allows the researcher to determine whether the results of the experiment are consistent with the expected results. If the results differ significantly from the theoretical values, it may be necessary to identify the source of the error and to repeat the experiment.
Overall, the discussion section of a titration lab report is an important part of the experimental process, as it allows the researcher to interpret and analyze the results of the experiment, identify sources of error, and compare the results to theoretical values. By carefully considering and discussing the results of a titration experiment, researchers can ensure that the results are accurate and reliable, and can use this information to make informed decisions and draw conclusions.