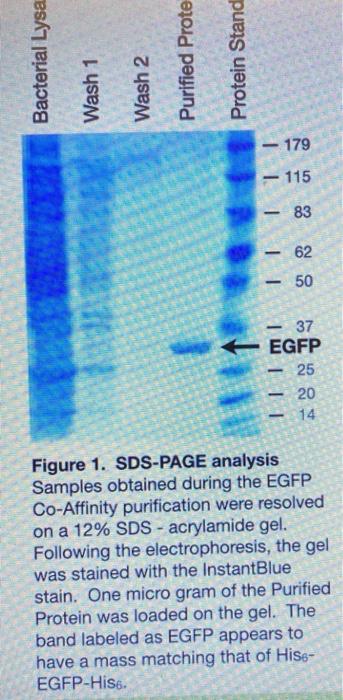
Protein purification is a critical step in many biotechnology and biochemistry laboratories, as it allows for the isolation and characterization of specific proteins for further study. In a protein purification lab report, it is important to thoroughly describe the methods used, the results obtained, and any relevant conclusions that can be drawn from the experiment.
The first step in a protein purification experiment is often the preparation of a sample containing the protein of interest. This can involve the isolation of the protein from a natural source, such as a tissue or cell culture, or the expression of the protein in a host organism, such as bacteria or yeast. Once the sample is prepared, it is typically subjected to a series of separation and purification techniques to isolate the protein of interest.
There are many different methods that can be used for protein purification, including chromatography, centrifugation, and electrophoresis. Chromatography involves the separation of proteins based on their physical properties, such as size, charge, or hydrophobicity. Centrifugation uses the force of spinning to separate proteins based on their density, while electrophoresis separates proteins based on their charge and size.
In a protein purification lab report, it is important to thoroughly describe the specific techniques used in the experiment, including the type of chromatography or other method used, the conditions of the experiment, and any modifications or optimizations made to the protocol. It is also important to include a detailed description of the sample preparation and any sample cleanup steps taken.
The results section of a protein purification lab report should include detailed information on the yield and purity of the purified protein, as well as any additional characterization data, such as mass spectrometry or enzymatic activity assays. It is important to include a discussion of any challenges or difficulties encountered during the experiment, and to provide a detailed analysis of the data obtained.
Finally, the conclusion of a protein purification lab report should summarize the main findings of the experiment and provide any relevant insights or observations. This might include a discussion of the implications of the purified protein for further research, or any potential applications of the protein in the broader field.
In summary, a protein purification lab report should provide a thorough and detailed account of the methods, results, and conclusions of the experiment. By carefully documenting the steps taken and the data obtained, researchers can provide a valuable resource for other scientists interested in studying the same protein, and contribute to a deeper understanding of the biological processes involved.