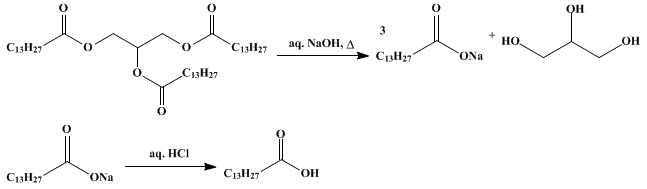
Trimyristin is a naturally occurring organic compound that is found in several plant sources, including nutmeg and mace. It is a triglyceride, which means that it is composed of three fatty acid chains attached to a glycerol molecule. Trimyristin is a solid at room temperature and has a melting point of around 46-47°C.
The melting point of a substance is the temperature at which it transitions from a solid to a liquid. It is a measure of the intermolecular forces within a substance, and is determined by the strength of the bonds between the molecules. In general, substances with strong intermolecular forces have higher melting points, while those with weaker intermolecular forces have lower melting points.
In the case of trimyristin, its melting point is influenced by the strength of the bonds between the fatty acid chains and the glycerol molecule. These bonds are relatively strong, which gives trimyristin a relatively high melting point. In addition, the three fatty acid chains in trimyristin are all 14 carbon atoms long, which also contributes to its high melting point.
Trimyristin has a number of uses in the food and cosmetics industries. It is used as a thickening agent in the production of margarine and other spreads, and is also used in the manufacture of soaps and other personal care products. It is also used in the production of certain fragrances and flavors.
In summary, trimyristin is a naturally occurring organic compound that is found in several plant sources and has a melting point of around 46-47°C. Its melting point is influenced by the strength of the bonds between the fatty acid chains and the glycerol molecule, as well as the length of the fatty acid chains. Trimyristin has a number of uses in the food and cosmetics industries and is used as a thickening agent, in the production of soaps and other personal care products, and in the production of certain fragrances and flavors.
3 Describe how your melting point for the trimyristin compares with the known

Names Appearance White-yellowish gray solid Odor Odorless Density 0. BOC Sciences is a brand of BOCSCI Inc. Tokyo Chemical Industry Co. The fatty acids and oils that are usually triesters are heated with sodium hydroxide. More than 70 years of synthesis experience and multi-purpose plants enable TCI to offer more than 30,000 products as well as custom synthesis. The literature gives a melting point range of 56-57 ° C for trimyristin. What are the theoretical properties of trimyristin? We leverage our wide spectrum of business in the fields of development, manufacturing, marketing, and distribution to help you make best-informed decisions tailored to your evolving needs for premium chemicals.
trimyristin For the pure trimyristin the melting point was 48C which is also

The percent yield of crude trimyristin from nutmeg was 49. As the second recrystallization was of the crude trimyristin product of the first recrystallization, not changes should have occurred, and the product can be identified as trimyristin. Ground nutmeg seeds are extracted with tert -butyl methyl ether and the resulting solid recrystallized from acetone to yield pure trimyristin. Why was it necessary to remove the solvent when isolating the trimyristin? The purpose of this experiment was to illustrate the process of obtaining a pure organic compound from a natural source. . After the recrystallization, a melting point between 55-56 ° C is achieved for the product. This range is between the values measured for each crude and pure myristic acid, demonstrating the concept of mixed melting points.
555
Email: US Email: Email: US Email: Voice: Moving Your Chemistry Forward We continuously strive to advance our technology. The range of melting points became narrower from the crude sample, to the first recrystallized sample, to the second recrystallized sample, most likely due to the decreasing amount of impurities following each recrystallization process. What is a good recovery percentage? Trimyristin comprises 73% of the fixed oil. The extraction is the first step to isolating the trimyristin from nutmeg and the product from the extraction was recrystallized to purify it. The product was recrystallized using acetone, and hydrolysis was performed to obtain myristic acid. Overall, trimyristin should have a percent recovery of 18-29%.
Trimyristin Melting Point
Use proper glove removal technique without touching glove's outer surface to avoid skin contact with this product. The fixed oil comprises approximately 24-40% of the nutmeg seed. Soap and Saponification - Chemistry accessed Oct 13, 2014. What is a good percent recovery? BOC Sciences provides a wide range of services to support the pharmaceutical industry through all stages of drug discovery including Custom Synthesis of those chemicals that are not in stock, Isotope Labeling Service, Chiral Synthesis and Resolution, Bioconjugation, PEGylation services, analytical services. The known melting point for trimyristin is 56 - 57 °C, which is similar to, but slightly higher than, the experimental melting points, possibly due to the presence of impurities.